Harvard Scientists Develop TDAC-seq, a Breakthrough Tool That Maps Gene Regulation at Single-Nucleotide Resolution
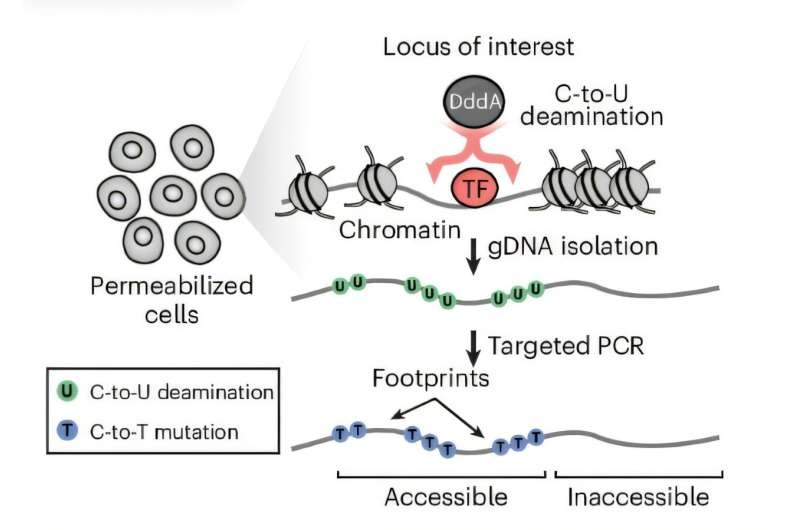
A team of researchers at Harvard University has unveiled a groundbreaking tool called TDAC-seq (Targeted Deaminase Accessible Chromatin sequencing) that can map how genes are regulated with extraordinary precision—right down to individual DNA bases. Published in Nature Methods in November 2025, this new technology could completely transform how scientists study genetic regulation and develop treatments for genetic diseases.
A New Window Into How Genes Are Controlled
For decades, scientists have known that understanding how genes turn on and off is key to unlocking the secrets of life. While only about 2% of our DNA codes for proteins, the remaining 98%—the so-called noncoding DNA—plays a massive role in regulating when, where, and how those proteins are made. This noncoding DNA is often referred to as the “dark matter” of the genome because it’s been notoriously difficult to study.
That’s where TDAC-seq comes in. The technique allows scientists to see how tiny genetic changes—even a single base pair—affect the structure of chromatin, the packaging that determines which genes are accessible to a cell’s machinery. Chromatin acts like a control panel for gene expression: when it’s open, genes can be activated; when it’s tightly packed, they stay silent.
The breakthrough came from Brian Liau’s lab at Harvard’s Department of Chemistry and Chemical Biology. Working with collaborators including Jason Buenrostro, a leading expert in chromatin accessibility, the team managed to pair genome editing with direct chromatin analysis in a way that’s never been done before.
How TDAC-seq Works
The innovation centers on a bacterial enzyme known as DddA, which has a unique ability: it can mark open sections of DNA by converting the base cytosine (C) into thymine (T)—and it does this without breaking the DNA strand.
The researchers took advantage of this property to develop a new method that layers CRISPR-based editing with deaminase-induced marking. Here’s how it works:
- Scientists use CRISPR to introduce hundreds of genetic changes across different parts of the genome, especially within noncoding regions.
- The DddA enzyme then identifies which sections of DNA are “open” and accessible to cellular machinery by converting cytosine to thymine at those spots.
- Using long-read sequencing—a technology that reads DNA molecules in large continuous stretches—TDAC-seq captures both the introduced genetic edits and the DddA-induced marks.
- The result is a single-nucleotide-resolution map showing how each change affects chromatin accessibility in real cells.
Because the sequencing is done on long molecules rather than fragmented DNA, the researchers can trace how specific edits influence nearby regions—something other techniques simply can’t do.
Why It’s a Big Deal
Before TDAC-seq, most genome-mapping tools gave only a broad overview of chromatin structure. Methods like ATAC-seq and DNase-seq reveal which DNA regions are open, but they don’t show how particular mutations or edits change chromatin accessibility on the same molecule.
TDAC-seq, on the other hand, offers single-molecule, single-nucleotide precision. This allows researchers to directly connect specific genetic changes to structural effects in the DNA. In essence, it shows the exact cause-and-effect relationship between DNA mutations and gene regulation.
That level of detail could have huge implications for medical research. Many diseases—including cancers, autoimmune disorders, and blood diseases—are linked to variants in noncoding regions. By revealing how those variants affect chromatin and gene expression, TDAC-seq could guide the development of new therapies that target gene regulation instead of just genes themselves.
Putting TDAC-seq to the Test
To demonstrate TDAC-seq’s capabilities, the team focused on a particularly important case: the regulation of fetal hemoglobin (HbF). This gene is a major player in diseases such as sickle cell disease and beta-thalassemia, where the body produces abnormal hemoglobin.
Using human blood stem cells, the researchers introduced DNA variants into regions that control fetal hemoglobin expression. They then applied TDAC-seq to measure how each change affected chromatin accessibility around those regulatory elements.
The results were impressive. The team could track the molecular consequences of each DNA alteration and see exactly how it modified chromatin structure. This allowed them to identify which variants increased HbF production—information that could directly support more effective gene-editing treatments for blood disorders.
In another experiment, the researchers applied TDAC-seq to study hundreds of noncoding variants in a regulatory enhancer of GFI1B, a gene tied to blood cell development and certain cancers. By examining how each variant reshaped chromatin structure, they were able to pinpoint key DNA sequences that act as molecular “switches.”
Overcoming Technical Hurdles
Developing TDAC-seq wasn’t easy. Early on, the team faced major challenges in getting the DddA enzyme to produce enough detectable mutations without damaging the DNA. Through careful optimization—testing different variants of DddA and adjusting reaction conditions—they managed to reach a mutation rate high enough to read chromatin accessibility reliably.
The researchers also had to tackle the massive data analysis problem. Because TDAC-seq generates long and information-rich DNA reads, standard analysis pipelines didn’t work. Graduate students Heejin Roh and Simon Shen, along with postdoctoral researcher Hui Si Kwok, built custom computational tools to interpret the data at single-molecule precision.
The Broader Impact
Beyond specific diseases, TDAC-seq opens up a completely new way to study the noncoding genome. For decades, scientists have been puzzled by how seemingly “silent” DNA can control the activity of genes far away on the chromosome. With TDAC-seq, it’s now possible to see these effects in direct molecular detail.
The technique also has major potential in functional genomics screens. Because it can handle pooled CRISPR experiments—where hundreds of edits are tested simultaneously—TDAC-seq allows researchers to explore thousands of regulatory variants at once. This scalability makes it a valuable tool for large-scale studies of gene regulation.
Looking ahead, the Liau Lab plans to refine TDAC-seq so it can work efficiently in even more cell types, including neurons and stem cells from different tissues. The goal is to make it a universal platform for studying how genetic changes shape gene activity and disease risk.
Why This Matters for the Future of Medicine
What makes TDAC-seq so exciting is its potential to bridge the gap between genetic variation and disease mechanism. Genome-wide association studies (GWAS) have identified thousands of DNA variants linked to diseases, but most lie in noncoding regions, making it unclear how they actually cause harm.
With TDAC-seq, researchers can test those variants directly, observe their molecular effects, and understand the biological pathways they disrupt. That could lead to the discovery of new drug targets and gene therapy strategies that are far more precise and effective than current approaches.
In conditions like sickle cell disease, where reactivating fetal hemoglobin can relieve symptoms, TDAC-seq provides a detailed view of which genetic edits truly open up the right chromatin regions to boost HbF levels safely.
The Future of Gene Regulation Research
The development of TDAC-seq represents a leap forward in our ability to read the language of the genome. It combines the power of CRISPR editing, enzymatic DNA marking, and long-read sequencing into a single workflow that connects cause (a mutation) to effect (a change in chromatin structure).
As this technology spreads, it could accelerate discoveries across multiple areas of biology—from cancer epigenetics and stem cell differentiation to neurogenomics and rare disease research. For the first time, scientists can systematically map how the invisible switches of the genome work, base by base, molecule by molecule.
If understanding gene regulation was once like looking at Earth from space, TDAC-seq brings the view down to the street level—where every nucleotide matters.





