New Technique Turns Everyday Plastics into Highly Porous Materials
Chemists at the University of Florida (UF) have unveiled a fascinating method that transforms simple plastics—those we often think of as waste—into high-performance porous materials. What’s truly interesting is that the secret lies not in what they add to the plastic, but in what they take away.
How the process works
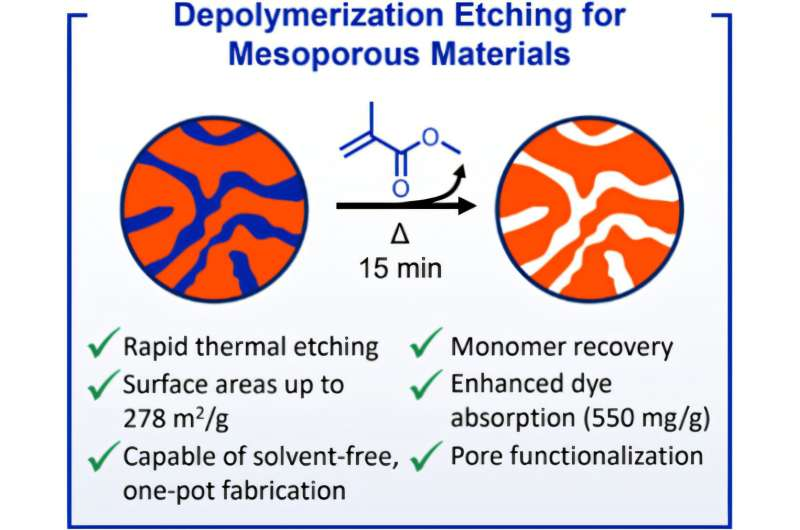
The team, led by Brent Sumerlin, developed a manufacturing technique that starts with a mix of two different polymer components: one that remains stable and intact under heat, and another that is engineered to break down and evaporate when heated. This internal “subtraction” creates a network of microscopic pores inside the remaining polymer scaffold.
Here’s the sequence in more detail: first, the researchers form a polymer matrix using a block-copolymer system in which microphase separation (distinct domains of different polymer types) occurs during polymerization. The stable domain is a cross-linked polystyrene-type matrix (resistant to thermal breakdown), while the etchable domain is made from a methacrylate-type polymer that upon heating can revert (depolymerize) to its monomer or gaseous products. (ACS Publications)
Once the material is formed, heating causes the etchable part to ‘disappear’ (via depolymerization) and leave behind tiny voids or pores in the solid matrix. Because the removal is internal and gas-driven, the method avoids many of the diffusion and solvent-limitations present in classical porous polymer fabrication. (ACS Publications)
What the results show
The porous materials developed through this “etch from within” strategy achieved surface areas exceeding 200 m² per gram in some cases. (ACS Publications) That’s an impressive figure for polymer-based materials. A sample weighing just a gram was reported to contain within its pores the equivalent surface area of a full-sized tennis court.
The technique can also be scaled up: the authors demonstrate a one-pot, gram-scale process where the materials are produced in under 12 hours. (ACS Publications) Because the etchable domain becomes gaseous, the pore formation is more uniform and rapid compared with solvent-based removal.
Why this matters
There are three key advantages to highlight:
- Scalability & simplicity: Many porous polymer manufacturing methods require complex solvent systems or lengthy processing. This method sidesteps many of those barriers by using thermal depolymerization.
- High surface area + structural integrity: The stable domain holds form while the etchable domain leaves behind high porosity. That combination is highly valuable in applications like filtration, separation membranes, electrodes in batteries etc.
- Sustainability crossover: Interestingly, this technique originates from the realm of plastic recycling/depolymerization research. But instead of just breaking plastics down into monomers for reuse, it uses the concept of depolymerization as a design tool for creating advanced materials. In other words, waste plastics or standard plastics might become the feedstock for high-value porous materials.
Potential applications
What could these materials be used for? The researchers point to several promising directions:
- Filtration and separation: Because the pore networks are highly interconnected and high in surface area, the materials could act as membranes or filters for water purification, gas separation, or other chemical separations.
- Energy storage and batteries: Porous scaffolds are valuable in electrodes, where high surface area and good ionic transport pathways enhance performance.
- Electronics or magnetic storage: The team suggests that with slight tweaks the materials could be adapted for high-density electronic or magnetic storage technologies, thanks to the finely controlled internal structure.
- Adsorbents: In their experiments they functionalised the porous polymer surfaces and showed they had high dye-adsorption capacities—meaning they could capture waste contaminants or be used in purification. (ACS Publications)
Some details worth noting
- The method is called Depolymerization Etching of Polymerization-Induced Microphase Separations (DEPIMS). (ACS Publications)
- The key to selectivity lies in the difference in depolymerization behaviour: for example, when a thermally labile comonomer (such as N-(methacryloxy)phthalimide) is incorporated into a poly(methyl methacrylate) (PMMA) system, more than 90% of the polymer can revert to monomer at ~290 °C in ~15 minutes. In contrast, a similar incorporation into a polystyrene backbone produced <10% reversion under the same conditions. This difference underpins the selective removal of one domain while the other remains intact. (ACS Publications)
- While 250-300 °C is required for these transformations, this is significantly lower than many traditional polymer pyrolysis or etching processes (which may require 400-600 °C) and hence less damaging to structure. (ACS Publications)
- The morphology engineered via polymerization-induced microphase separation (PIMS) allows them to preset the domain sizes, volumes and therefore final pore size distributions. That means the approach gives control—not just random porosity. (Western Sydney University Researchers)
Some caveats and things to watch
- The elevated temperatures (250-300 °C) may limit some substrates or combine poorly with heat-sensitive components or additives.
- The materials system needs both a domain that can depolymerize readily and a domain that resists breakdown—so the polymer chemistry must be carefully designed.
- Though gram-scale demonstration is reported, industrial scale-up (tons scale, integration into commercial devices) will require further engineering and validation.
- For certain applications, the exact pore size distribution or connectivity may need further tuning—while the method shows high surface area, for some highly specialised purposes (e.g., ultrafiltration, gas separation) fine tuning may still be required.
Why this matters for the bigger picture
In a world that increasingly demands materials that are both high-performance and sustainable, this technique checks a lot of boxes. It re-imagines how we view plastics—not just as waste to break down but as building blocks. By carving (“sculpting”) from within, using thermal depolymerization to etch porosity, the team has merged recycling insights with advanced materials fabrication.
For researchers and industry, this opens up a new paradigm: instead of relying solely on additive manufacturing, composite layering or nanoparticle infusions to achieve porosity, one can program the material from the start such that the internal microstructure is built-in and then selectively carved out.
What you might want to ask next
If you were following how this evolves, some questions to keep an eye on could include:
- Will this method be adapted for other polymer systems (beyond styrene or methacrylate systems)?
- Can the process be integrated with existing battery electrode manufacturing lines or water-filter membrane production?
- What are the costs and energy requirements for the thermal step compared with traditional manufacturing?
- Could recycled plastics themselves serve as the starting block (feeding this technique directly from waste streams)?
- How tunable are the pore sizes really—down to nanometres for specific separation tasks?
Reference:
Kaden C. Stevens, Megan E. Lott, Kiana A. Treaster, Robert M. O’Dea, Adarsh Suresh, Cabell B. Eades, Victoria L. Thompson, Jared I. Bowman, James B. Young, Austin M. Evans, Stuart J. Rowan, Thomas H. Epps III & Brent S. Sumerlin. Depolymerization as a Design Strategy: Depolymerization Etching of Polymerization-Induced Microphase Separations. ACS Central Science 2025, DOI: 10.1021/acscentsci.5c01313. (ACS Publications)