Bacterial Enzyme Unlocks a New Route to Renewable Plastics
Scientists have uncovered a remarkable enzyme called methylthio-alkane reductase (MAR) that could change how we make one of the most common building blocks for plastics — ethylene. Unlike the traditional fossil-fuel method that releases massive amounts of CO₂, this enzyme works under oxygen-free conditions and avoids that CO₂ by-product entirely.
What’s the discovery?
Researchers working with the soil bacterium Rhodospirillum rubrum found that MAR can produce ethylene from volatile organic sulfur compounds (VOSCs) when there’s no oxygen around. These VOSCs include molecules like methylthio-ethanol (MT-EtOH) and dimethyl sulfide (DMS). In the reaction, the enzyme cleaves a carbon–sulfur (C–S) bond and generates methanethiol (for sulfur metabolism) and small hydrocarbons such as ethylene, ethane, or methane. (Nature)
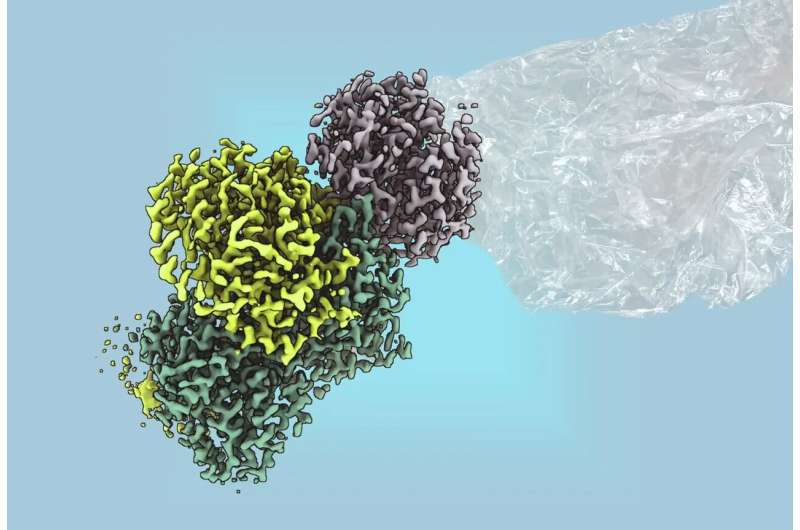
What’s more, the team managed to isolate and purify MAR, determine its structure (via cryo-EM and other methods) and show exactly how it works down to the metal-cluster level. (X-MOL)
Why this matters
Ethylene is a fundamental feedstock used to make plastics (like polyethylene), packaging materials and many other chemical products. Traditionally it’s derived from cracking fossil hydrocarbons, which uses lots of energy and emits CO₂. MAR offers a potential biological production route under milder conditions, using bacteria + enzyme rather than fossil fuel + high heat + large CO₂ emissions. (Engineeringness)
On the biological side, MAR is particularly exciting because it uses large iron-sulfur metalloclusters that were previously thought to only exist in enzymes like nitrogenases (which fix atmospheric nitrogen). That means MAR bridges our understanding of enzyme evolution, metal cluster chemistry and renewable chemical production. (Nature)
Key technical details
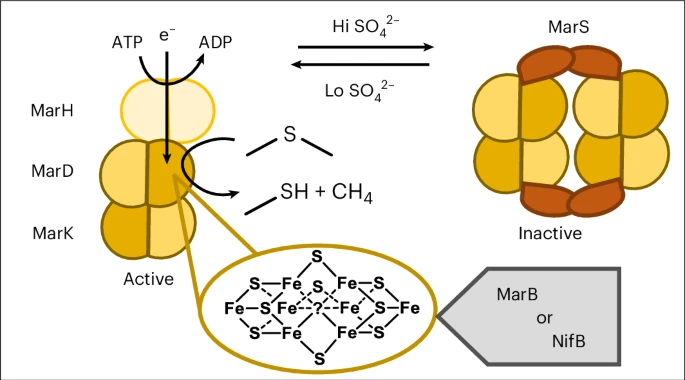
- MAR is a two-component enzyme system, involving a reductase (MarH) and a catalytic core (MarDK). Together they act on VOSCs to release methanethiol and hydrocarbons. (Nature)
- The structural work uncovered that the catalytic core carries metalloclusters akin to the P-cluster and [Fe₈S₉C] cluster found in nitrogenase systems. This is the first time such clusters are seen in a non-nitrogen-fixing enzyme. (MPG.PuRe)
- Activity requires anaerobic conditions, ATP (energy), and an electron donor— just like nitrogenase-type enzymes. (Nature)
- Substrates: For example, MT-EtOH (2-methylthio-ethanol) can be converted into methanethiol plus ethylene; DMS can yield methane. The reaction is stoichiometric: For each C–S bond cleaved you get one small hydrocarbon out. (Nature)
- The regulation: MAR is triggered when sulfate (a common inorganic sulfur source) is scarce. In other words, the bacterium switches to VOSC-based sulfur scavenging via MAR under certain conditions. (Nature)
Broader implications
For plastics & industrial chemistry: If biotechnologists can harness or engineer MAR (or an improved version) to work at scale, we might someday make ethylene biologically from renewable or waste sulfur compounds, reducing dependence on fossil fuels and lowering CO₂ emissions. The fact that MAR doesn’t release CO₂ in the conversion is a big plus.
For enzyme evolution & bioinorganic chemistry: The discovery that MAR uses large iron-sulfur clusters (once thought limited to nitrogenases) suggests that these “great clusters of biology” have broader roles than previously assumed. It implies a possible ancient evolutionary lineage of enzymes using such clusters for diverse reductive chemistries—even before nitrogen fixation evolved. (Phys.org)
For microbial metabolism & ecology: VOSCs are abundant in many environments (e.g., wetlands, certain soils) and are part of the global sulfur cycle. MAR offers a mechanism by which bacteria can use these compounds for sulfur acquisition, producing small hydrocarbons in the process. That has interesting ecological and geochemical implications. (Nature)
What remains to be done
While this is a big step forward, several challenges remain before MAR becomes industrially useful:
- MAR is oxygen-sensitive, meaning it must operate in strict anaerobic conditions. That complicates engineering for large-scale use.
- We need to improve catalytic efficiency, substrate specificity and stability of the enzyme and the host organism.
- The current natural substrates (VOSCs) may not be ideal as feedstocks for large-scale production; one needs cheaper, abundant starting materials or efficient conversion of waste streams.
- Integration into a full process: The enzyme must be embedded in a microbial host (or cell-free system) with electron supply, feedstock supply, product extraction, all at scale.
- Environmental and economic viability must be assessed: It’s one thing to show it works in the lab; it’s another to make it cost-competitive and sustainable at industrial scale.
Extra background: Iron-Sulfur Clusters & Enzyme Families
What are iron-sulfur clusters? These are groups of iron and sulfur atoms that act as cofactors inside many enzymes, helping shuttle electrons or participate in catalysis. They’re ancient, and very common in biological redox chemistry.
What is the nitrogenase family? Enzymes in this family (e.g., in nitrogen-fixing bacteria) reduce dinitrogen (N₂) to ammonia (NH₃), enabling life to access atmospheric nitrogen. They carry big metal clusters like the P-cluster and FeMoco, and operate under strict conditions (often anaerobic). MAR shares structural resemblance with these, but performs a different chemistry (C–S bond cleavage instead of N₂ reduction).
Why does this matter? Because it broadens our view of what such complex metallocluster enzymes can do. Instead of being specialized only for nitrogen fixation, they may have diversified to catalyse hydrocarbon formation, sulfur extraction, even hydrocarbon release. That opens new fields for enzyme engineering and novel biocatalysts.
Final thoughts
The discovery of MAR and its detailed structural/functional characterisation is exciting for multiple reasons. It points to a potential renewable route to a key plastic precursor (ethylene), it expands our understanding of enzyme evolution and metal cluster chemistry, and it highlights a clever microbial strategy for sulfur acquisition with side-benefits of hydrocarbon release.
If engineered and optimised further, MAR or related enzymes might help reduce our reliance on fossil-derived ethylene and move us closer to more sustainable plastic production. At the same time, the knowledge gained about how metal clusters work in proteins is likely to be useful in many other catalytic and synthetic biology applications.
Here’s the research paper:
Murali, S., Hu, G.-B., Kreitler, D. F., Arroyo Carriedo, A., Lewis, L. C., Fosu, S. A., … North, J. A. (2025). Architecture, catalysis and regulation of methylthio-alkane reductase for bacterial sulfur acquisition from volatile organic compounds. Nature Catalysis. DOI: https://doi.org/10.1038/s41929-025-01425-3