How Optical Brightness of Battery Particles Reveals the True State of Charge
Researchers at Purdue University have uncovered a remarkably accessible way to understand what’s really happening inside lithium-ion batteries as they charge. Instead of relying on expensive, high-powered imaging tools, they’ve shown that something as simple as a basic optical microscope and an RGB camera can reveal how individual particles inside a battery behave. This can help scientists better diagnose battery performance, safety, and long-term health by observing the brightness of the tiny particles that make up a battery’s electrodes.
Their work centers on a fascinating discovery: the micro-scale particles inside a battery’s electrode actually become brighter as they charge. By tracking these brightness changes across hundreds of particles at once, the team was able to reconstruct a highly accurate mathematical picture of how evenly the battery charges.
This research was published in the Proceedings of the National Academy of Sciences and focuses on one fundamental challenge in battery science—heterogeneity, or how unevenly charge is distributed across the many particles that form an electrode. That unevenness can have a major impact on performance and even lead to serious safety issues.
Below is a detailed, straightforward breakdown of the research, the methods, the findings, and why this matters for the future of battery technology.
The Experiment Setup and What the Researchers Did
The Purdue team worked with lithium-ion coin-cell batteries, assembling and testing them inside a glove box filled with inert gas to avoid unwanted reactions (lithium is highly reactive in open air). Inside the cathode of these batteries are lithium nickel manganese cobalt oxide (NMC) particles—small metal-oxide grains that store and release lithium during charging and discharging.
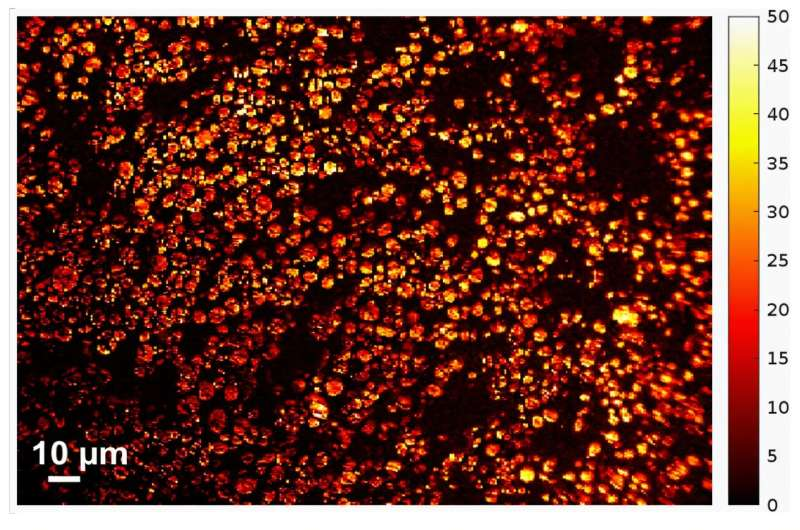
To examine these particles, the researchers pointed a standard optical microscope at a small region containing 100 to 1,000 individual particles. They then slowly charged the battery and recorded time-lapse videos of the same particles over several hours. No special filters, ultra-precise focusing, or advanced optics were required.
What they discovered is surprisingly elegant: as each particle becomes more charged, it also becomes optically brighter. This brightness shift is consistent and measurable, allowing it to serve as a direct visual indicator of how much charge a particle holds.
With image-processing tools, they analyzed every particle’s brightness frame by frame. From this, they could reconstruct a spatial map showing how evenly or unevenly the battery was charging at any given moment. Areas with brighter particles indicated higher charge, and darker areas indicated slower or incomplete charging.
Why Particle-Level Brightness Matters
A battery’s performance is not only about its overall charge level. It also depends heavily on how evenly charge moves through the electrode. If particles near one region of the electrode charge faster than others, it can lead to:
- Localized degradation
- Reduced capacity over time
- Formation of defects
- Hot spots
- Thermal runaway, a dangerous failure mode where the battery overheats uncontrollably
The Purdue researchers found that analyzing brightness allowed them to quantify heterogeneity more precisely than many existing methods. They also showed a strong mathematical correlation between optical brightness and the actual electrochemical state of charge.
This means brightness can serve as a reliable, low-cost diagnostic signal for many types of battery materials—not just NMC, but also lithium cobalt oxide, graphite, and others whose electrical and optical properties change during charging.
The Scale of Heterogeneity and What It Tells Us
One of the most important insights from the study is identifying the scale at which heterogeneity becomes meaningful. The team found that to understand a battery’s overall behavior, you need to observe a group of roughly 100 to 1,000 particles. Below that number, the data becomes too localized to represent the full electrode; above it, you begin to see diminishing returns.
This finding helps battery researchers determine what size of sample region provides a truly representative picture. It also supports the idea that even small inconsistencies at the particle level can cascade into large-scale behavior differences.
Why This Approach Is a Breakthrough
Until now, observing active particles during charging often required:
- High-powered X-ray imaging
- Synchrotron facilities
- Electron microscopy
- Specialized coating and beamline techniques
These tools are expensive, difficult to access, and not practical for routine industrial use.
In contrast, the Purdue approach uses readily available optical equipment—the kind found in many labs and even educational institutions. The researchers also demonstrated that their results remain valid even when the image is slightly out of focus. That makes this technique:
- Cost-effective
- Scalable
- Fast
- Practical for industry
- Non-invasive
It opens the door to real-time, in-factory quality control that monitors how particles behave as the electrode is manufactured and assembled.
What Causes Battery Heterogeneity?
Battery electrodes are a mixture of:
- Active particles (like NMC)
- Conductive carbon
- Polymer binders
- Pores filled with electrolyte
This composite structure isn’t perfectly uniform. Differences in particle size, shape, contact quality, or connectivity can lead to uneven charging paths. A particle poorly connected to conductive additives will charge more slowly. A cluster of particles with stronger electronic pathways may overcharge relative to others.
These local variations create complex networks of non-uniform behavior, which can only be understood by looking at many particles simultaneously—something this new optical approach makes possible.
What This Means for Battery Safety and Longevity
Understanding heterogeneity is essential for:
- Predicting degradation rates
- Designing safer battery systems
- Improving fast-charging performance
- Extending cycle life
- Preventing catastrophic failures
By mapping brightness over time, manufacturers could detect:
- Regions with poor particle connectivity
- Overactive or underperforming particle clusters
- Structural inconsistencies that may lead to cracks or defect growth
With early detection, interventions can be made long before the battery reaches the consumer.
Broader Implications for Future Battery Technologies
Although the study focused on NMC materials, the authors note that brightness-based characterization works for many electrode chemistries. This opens up potential applications across:
- Electric vehicle batteries
- Grid-scale storage
- Consumer electronics
- Next-generation solid-state batteries
This technique could also guide future materials research by making it easier to test how new electrode designs behave under real charging conditions.
Additional Background: Why Lithium-Ion Particles Change Brightness
When a lithium-ion battery charges, lithium ions move out of the cathode particles and into the anode. This change affects:
- Electrical conductivity
- Optical reflectivity
- Structural arrangement of atoms
In many metal-oxide materials, the reduction in lithium content changes how electrons interact with light, making the particles appear brighter. This optical shift, once considered insignificant, is now recognized as a powerful diagnostic tool.
Research Reference
Proceedings of the National Academy of Sciences – On the Scale of Heterogeneity in Composite Electrodes of Batteries
https://doi.org/10.1073/pnas.2520136122