UC Berkeley Team Develops a New Lipid Nanoparticle That Delivers mRNA Directly Into Human Heart Muscle Cells

A new advance in heart-focused gene therapy may finally help solve a problem that has slowed progress in cardiovascular treatments for years: the inability to reliably deliver therapeutic mRNA into actual heart muscle cells, also known as cardiomyocytes. A research team led by UC Berkeley, in collaboration with the Gladstone Institutes and UCSF, has identified a new type of lipid nanoparticle (LNP) that can penetrate dense heart tissue and release its mRNA cargo directly into cardiomyocytes with much higher efficiency than existing formulations.
Their findings were published in Nature Biomedical Engineering, and the details reveal why this development could matter for future treatments aimed at heart failure, cardiomyopathies, gene correction and other cardiac disorders.
A New Approach to an Old Delivery Problem
Cardiovascular disease remains the leading global cause of death, yet progress in heart-failure therapeutics has slowed.
One major bottleneck is the difficulty of delivering therapies—especially genetic or molecular therapies—deep into the thick, tightly packed muscle cells of the heart. Unlike the liver or spleen, the heart is far harder for nanoparticles to infiltrate. And even when they make it inside a cell, they often fail a critical step called endosomal escape, which determines whether the therapeutic mRNA actually reaches the cytoplasm where it is needed.
Lipid nanoparticles are tiny, fat-based spherical particles that encapsulate and transport sensitive molecules like mRNA. They are currently the most clinically advanced non-viral delivery systems for mRNA therapies, used prominently in Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines.
But delivering mRNA to cardiomyocytes requires more than just a stable nanoparticle. Once taken into the cell, most mRNA remains trapped in endosomes—cellular compartments that act like sorting stations. If the nanoparticle cannot escape quickly enough, the mRNA degrades before achieving any therapeutic effect.
The UC Berkeley–led team specifically targeted this issue by engineering a nanoparticle that not only diffuses into heart muscle more easily but also breaks free from the endosome once inside the cell.
The Heart-on-a-Chip That Made Discovery Possible
A core innovation behind this research is the use of a human cardiac microphysiological system (MPS)—a sophisticated heart-on-a-chip device. This miniature model of the human heart includes 3D micromuscles grown from living human cells and microfluidic channels thinner than a human hair.
By controlling fluid flow, oxygen, and biochemical conditions, the device closely mimics the environment of actual heart tissue, offering far more realism than traditional two-dimensional cell cultures.
These 3D tissues recreate the tight, dense structure of real cardiomyocytes, making them much better predictors of how therapies will behave inside the body. The researchers tested multiple LNP formulations in this system, allowing them to identify which nanoparticles most effectively penetrated heart tissue and delivered functional mRNA.
A New Kind of Lipid Nanoparticle
The researchers created LNP candidates with a novel acid-degradable polyethylene glycol (PEG) coating. PEG coatings help nanoparticles stay stable and travel through tissues, but they often prevent endosomal escape. The team’s solution was to design a coating that breaks apart in acidic environments—like those found inside endosomes—allowing the mRNA to be released at the right time.
This acid-degradable PEG coating allowed the LNPs to remain stable while traveling through dense heart tissue but also ensured that once inside cardiomyocytes, the nanoparticle could shed the coating and successfully release its mRNA into the cytoplasm.
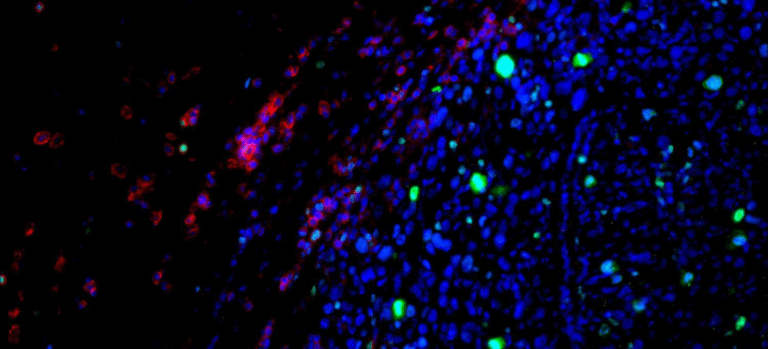
In their heart-on-a-chip experiments, the team tested multiple iterations of these nanoparticles, assessing diffusion, uptake, and mRNA release. The most successful formulation showed strong penetration into cardiac muscle fibers and high levels of mRNA expression in the cardiomyocytes.
Testing in Mice Validates the System
To confirm that the in vitro results reflected real-world performance, the researchers then tested the same optimized nanoparticles in mouse hearts. The results matched the predictions from the heart-on-a-chip models: the acid-degradable PEG LNPs efficiently delivered their mRNA cargo directly into heart muscle cells.
Just as importantly, the mice showed reduced off-target delivery to the liver, which is a common unintended destination for many nanoparticle therapies. This supports the idea that the heart-on-a-chip system can meaningfully predict in vivo behavior and may reduce the number of animal studies needed during drug development.
Why This Matters for mRNA Cardiac Therapies
This discovery opens the door to potential new treatments for heart disease that rely on mRNA or gene-editing tools. With a delivery system that works reliably in heart muscle, scientists can more confidently develop therapies that repair genetic defects, promote regenerative processes, or strengthen failing heart tissue.
It also helps solve one of the most persistent problems in the field: the gap between early laboratory tests and success in live organisms. Traditional 2D cell cultures almost never predict what will happen in complex tissue environments, causing many promising therapies to fail in animal or clinical studies. A 3D system that accurately reflects human tissue behavior offers a more efficient and humane pathway for therapeutic development.
More About Lipid Nanoparticles
Because this study centers on lipid nanoparticles, it’s helpful to understand what makes them important and how they work.
What Exactly Is a Lipid Nanoparticle?
LNPs are tiny structures made from ionizable lipids, helper lipids, cholesterol, and PEG-lipids. They protect and transport fragile genetic material, like mRNA or siRNA, through the bloodstream. Their lipid components merge with cellular membranes, allowing the cargo to slip inside.
Why the PEG Coating Matters
PEG provides stability and prevents immune clearance, but too much PEG or the wrong type of PEG can prevent the particle from entering cells efficiently. The team’s acid-degradable PEG is uniquely clever because:
- It secures the nanoparticle during circulation
- It detaches inside the acidic endosomal environment
- It improves the odds of successful endosomal escape
- It reduces destruction of the mRNA payload
This type of PEG design could also influence delivery systems for other hard-to-reach organs like the brain, lungs, or pancreas.
Endosomal Escape: The Critical Step
When nanoparticles enter cells, they do so inside vesicles called endosomes. If they remain trapped, the mRNA cargo breaks down. Successful delivery requires the nanoparticle to exit the endosome and reach the cytoplasm. This is one of the most challenging engineering problems in drug delivery, and any improvement—like the one shown here—can dramatically increase therapeutic effectiveness.
Implications for the Future of Heart Treatments
The combination of a predictive heart-on-a-chip platform and a more effective nanoparticle suggests a faster pathway toward developing heart-targeted mRNA therapeutics. Researchers can now screen countless formulations without the heavy time and cost burden of animal testing. This can dramatically accelerate early-stage drug development and may encourage innovation in cardiac gene therapy.
Eventually, therapies that repair genetic mutations linked to cardiomyopathies, or that help regenerate damaged heart tissue after a heart attack, could come from this kind of technology.
Research Reference
Nature Biomedical Engineering – A microphysiological system for screening lipid nanoparticle–mRNA complexes predicts in vivo heart transfection efficacy
https://doi.org/10.1038/s41551-025-01523-4