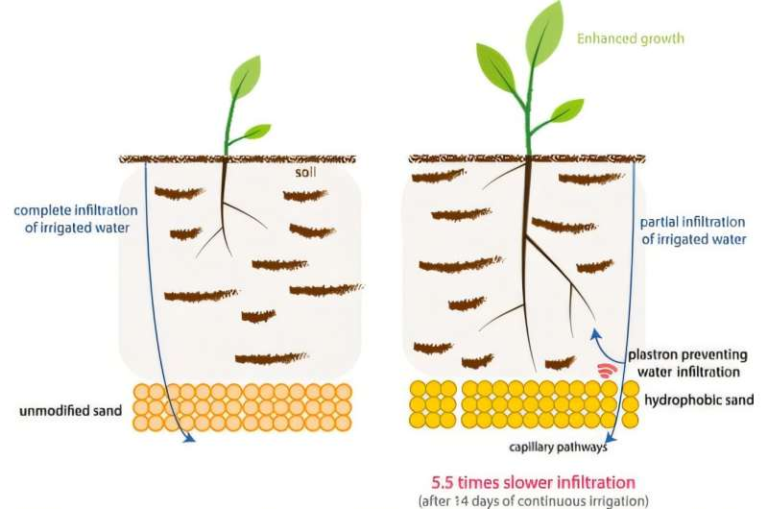
How New Algorithms Reveal the Atomic Secrets Behind Turning Propane Into Propylene
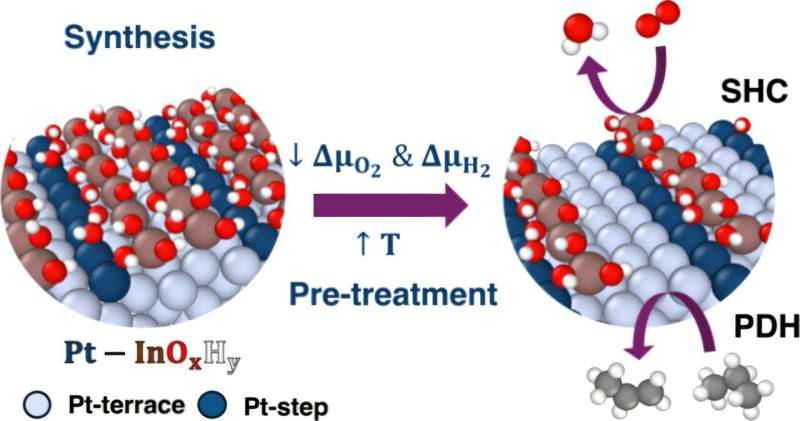
Researchers have taken an impressive step toward understanding one of the most important industrial chemical transformations: converting propane into propylene, the building block behind everyday products like plastic bottles, outdoor furniture, packaging materials, and countless household goods. A new study from the University of Rochester digs deeper than ever before into how this reaction actually works at the atomic level, thanks to a set of refined algorithmic tools designed to analyze extremely complex catalytic systems.
This is a big deal because although the industry produces massive amounts of propylene every year, the fine-grained mechanisms behind some of the most promising catalytic processes have remained unclear. Companies know these methods work, but not precisely why they work — and that lack of understanding limits innovation. This new research attempts to change that by delivering a detailed look at how specific catalytic materials behave under reaction conditions.
Below is a clear, direct breakdown of everything revealed in the new study, why it matters, and how it connects to the broader world of industrial chemistry.
A Clearer Look at How Propane Becomes Propylene
The study focuses on a catalytic approach known as tandem nanoscale catalysis, where different parts of the same catalyst perform separate but coordinated tasks. A major 2021 paper in Science showed that such tandem catalysts can combine multiple reaction steps into one, boosting propylene production while reducing costs and complexity. But researchers still lacked a precise atomic-level picture of how these materials behave.
The new work, published in the Journal of the American Chemical Society, provides exactly that. The research team developed and refined algorithms to systematically evaluate countless possible atomic configurations on a catalyst’s surface. This matters because catalytic reactions—especially ones involving both metal and oxide phases—are incredibly intricate. There are many potential arrangements of atoms, and each arrangement can influence the reaction differently.
By screening these possibilities, the team identified the key atomic features that drive the conversion of propane to propylene. One of the most notable findings is how the oxide layer in the catalyst behaves. The oxide does not simply form uniformly; instead, it grows selectively around defective metal sites. These defective sites are imperfect locations on the metal surface, and they turn out to play a crucial role in maintaining catalyst stability.
Another important detail is that even though the oxide can exist in multiple chemical compositions under reaction conditions, it consistently keeps its function of surrounding those defective metal sites rather than drifting away or reorganizing randomly. This selective behavior is essential for helping the catalyst maintain high performance.
Why Understanding the Atomic-Level Details Matters
Catalysts are at the heart of modern chemical manufacturing. They speed up reactions, reduce energy demands, and make large-scale production possible. For decades, companies have relied on trial-and-error to optimize catalytic systems. Tweaking materials, changing reaction conditions, and repeatedly testing results is slow, expensive, and often inefficient.
With the new algorithmic framework, researchers can start replacing trial-and-error with targeted catalyst design. For processes like turning propane into propylene — a multibillion-dollar industry — even small efficiency gains can have major industrial impact.
The new analysis also provides insights into stability, one of the biggest challenges in catalytic chemistry. Catalysts often degrade under harsh reaction conditions. When the researchers found that the oxide layer naturally prefers defective metal sites, they uncovered a built-in stabilizing effect. This understanding can help engineers create catalysts that last longer, resist deactivation, and deliver higher yields over time.
Exploring the Tandem Metal–Metal Oxide Catalyst
The catalyst studied in this work consists of a metallic phase and an oxide phase, which work in tandem during the reaction. The metallic phase handles the initial steps of breaking hydrogen atoms off the propane molecule. The oxide phase then helps manage those hydrogen atoms so they don’t interfere with the reaction or cause unwanted side reactions.
The algorithms allowed the researchers to analyze these two phases in great detail. They could see how each atomic site contributed to the overall reaction, what structures were most stable, and how the oxide rearranged as conditions changed. This ability to monitor atomic-scale transformations is extremely valuable, since such rearrangements often determine whether a catalyst succeeds or fails.
Importantly, the researchers discovered several aspects of the reaction that had not been predicted before — revealing just how complex and dynamic catalytic systems truly are.
How This Study Fits Into the Larger Story of Propylene Production
Propylene production is a massive industry because propylene is essential for manufacturing polypropylene, one of the most widely used plastics in the world. Traditionally, propylene is produced through energy-intensive methods such as steam cracking. These methods require extremely high temperatures and often generate significant byproducts.
The development of tandem nanoscale catalysts aims to create alternate pathways that:
- use less energy
- boost selectivity (producing more propylene and fewer unwanted compounds)
- reduce environmental impact
- lower industrial costs
To optimize these new catalytic pathways, scientists need clear insights into what is happening at microscopic and atomic scales. That’s exactly what the current research provides.
Potential Applications Beyond Propylene
While this study focuses on propane’s transformation into propylene, the implications reach far beyond this single reaction. According to the researchers, their algorithmic approach is general enough to be applied to many other important catalytic processes.
One example they highlight is methanol synthesis, a major industrial reaction used to produce chemicals, fuels, and various feedstocks. Understanding the atomic structure of catalysts used in methanol synthesis could lead to breakthroughs similar to those seen in propane-to-propylene chemistry.
Researchers also believe the same tools may help explain complex processes that have remained poorly understood for decades. As algorithms become more powerful and computational resources grow, such methods may eventually reshape how the chemical industry designs catalysts altogether.
Why This Research Is a Step Forward
Here are the core contributions from this study:
- It provides detailed atomic-scale insight into a tandem metal–metal oxide catalyst.
- It shows that oxide growth around defective metal sites is selective and crucial to catalyst stability.
- It reveals that the oxide maintains its role consistently, even across multiple chemical compositions.
- It introduces refined algorithms that can be used across various catalytic systems.
- It offers a pathway toward more efficient industrial production of propylene and potentially many other materials.
This combination of computational tools and experimental understanding represents a shift toward smarter, more informed catalyst design in industrial chemistry.
Additional Background: Why Propylene Matters
Propylene is used to make:
- food packaging
- automotive parts
- medical devices
- outdoor equipment
- household goods
- synthetic fibers
Its global demand continues to grow each year. Improving the way it is produced could have significant economic and environmental benefits. As industries look for cleaner and more efficient manufacturing methods, insights like those provided in this study become increasingly valuable.
Research Reference
Site-Selective Oxide Rearrangement in a Tandem Metal–Metal Oxide Catalyst Improves Selectivity in Oxidative Dehydrogenation of Propane
https://doi.org/10.1021/jacs.5c13571