How ARPC5 Deficiency Disrupts Cell Structure, Weakens Immunity, and Unbalances the Gut Microbiome
Researchers have long known that the internal skeleton of a cell—the cytoskeleton—is crucial for maintaining cell shape, movement, and responsiveness. At the heart of this structure lies actin, a protein that forms dynamic filaments and allows cells to change shape, migrate, and interact with their environment. A major driver of these actin networks is the Arp2/3 complex, and one of its essential components is a protein called ARPC5.
A new study published in Science takes a close look at what happens when ARPC5 is missing, and the findings reveal a surprisingly deep connection between immune cell structure, microbiome balance, and gut health. The research team from the Francis Crick Institute investigated how weaknesses in this structural protein can lead to systemic inflammation, sepsis, and breakdown of immune-microbe cooperation. The results show how critical this single protein is for maintaining bodily balance—especially right after weaning, when the microbiome shifts dramatically.
The Critical Role of ARPC5 in Immune Cell Function
ARPC5 belongs to the group of proteins that construct the branched actin networks essential for cell stability and movement. When this protein is mutated in humans, children often experience immunodeficiency and dangerous, early-life sepsis. Until now, the exact reason for this severe response wasn’t fully understood.
The new research fills that gap. The scientists studied mice with mutations in ARPC5 and compared them to healthy mice. Their goal was straightforward: understand how a structural defect inside immune cells leads to life-threatening illness.
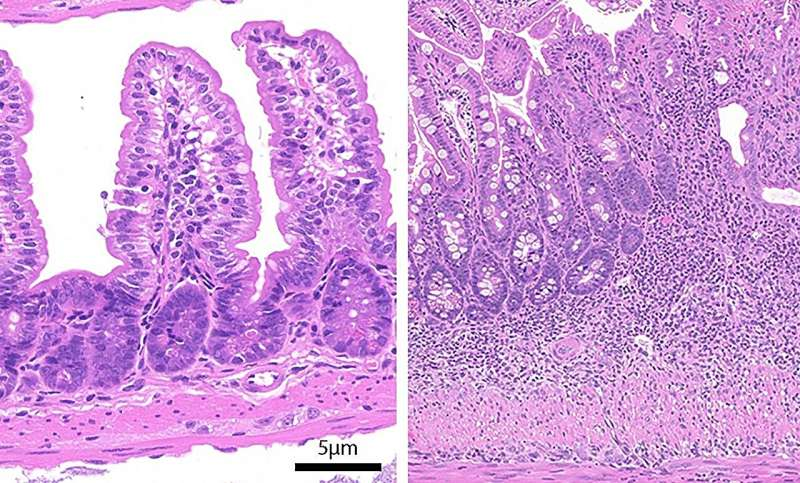
The findings were striking. Healthy mice showed normal gut structure and weight. But ARPC5-deficient mice displayed underdeveloped bodies, inflamed and damaged small intestines, and symptoms of systemic infection. These problems were particularly pronounced at eight weeks of age.
This age is important because it marks the period after weaning, when mice stop receiving immune protection from their mother’s milk and undergo a major shift in gut bacteria composition. At four weeks old—right before this microbiome transition—the mutant mice appeared completely normal. This timing revealed a key clue: the microbiome shift itself seemed to trigger the cascade of immune dysfunction.
How the Microbiome Triggers Disease in ARPC5-Deficient Mice
The gut microbiome changes dramatically after weaning, as mice transition from milk to solid food. This shift introduces new bacterial groups into the intestines and alters microbial balance. When researchers analyzed the microbiome of ARPC5-deficient mice before and after weaning, they confirmed that this transition coincided with the earliest signs of trouble.
To test whether the microbiome was truly responsible for triggering disease, the team administered antibiotics to ARPC5-deficient mice right at the four-week mark. The result was remarkable: antibiotic treatment completely prevented disease development. The mice did not develop inflamed intestines, did not become underweight, and did not show signs of sepsis.
This demonstrated that the interaction between immune cells and gut bacteria was the root cause of the inflammation. With ARPC5 missing, immune cells simply couldn’t handle the bacterial changes that normally occur during weaning.
What Goes Wrong Inside ARPC5-Deficient Immune Cells
To understand why ARPC5 deficiency has such dramatic effects, the scientists examined the structure and function of key immune cells—especially macrophages and regulatory T cells.
Their observations showed that macrophages lacking ARPC5 lose their normal rounded shape and become abnormally elongated. This deformation isn’t just cosmetic; it affects their ability to perform one of their most important jobs: engulfing and killing bacteria. Healthy macrophages rely on branched actin networks to reorganize their shape during phagocytosis. Without ARPC5, this process fails.
As a result, bacteria from the gut are able to infiltrate tissue, multiply, and even escape into the bloodstream, leading to inflammation and sepsis. Meanwhile, the crosstalk between macrophages and T regulatory cells, which normally helps maintain immune balance, breaks down. The immune system loses its steady state, and widespread inflammation takes over.
This breakdown explains why the disease appears only after weaning: the structurally compromised macrophages are unable to manage the sudden changes in microbiota introduced by the new diet.
Bone Marrow Transplantation Reverses the Damage
One of the most promising outcomes of the study is the discovery that bone marrow transplantation—essentially replacing faulty immune cells with healthy ones—reverses the inflammation in ARPC5-deficient mice. When the mice received healthy bone marrow, their immune function normalized, their gut inflammation cleared, and their vulnerability to infection decreased.
For people with ARPC5 mutations, this suggests that early bone marrow transplantation may be an effective treatment option. Replacing the defective immune cells could restore normal actin cytoskeleton function and prevent life-threatening infections.
Why This Discovery Matters Beyond Rare Genetic Disorders
While ARPC5 deficiency is a rare condition, the study offers far broader implications. Many immune and inflammatory diseases involve macrophage dysfunction, including inflammatory bowel disease (IBD). The research highlights how even subtle issues with cell structure can cascade into severe systemic dysfunction. It reinforces the concept that the cytoskeleton is not just a mechanical structure—it’s central to how immune cells sense, respond to, and control microbes.
Conditions where macrophages exhibit structural abnormalities may involve similar mechanisms of microbiome mismanagement. Understanding these connections opens new possibilities for diagnosing and treating diseases where immune cells fail to maintain harmony with gut microbes.
Understanding the Cytoskeleton: A Brief Overview
To give readers more context, it’s helpful to understand what the cytoskeleton is and why actin networks matter:
The Cytoskeleton’s Main Functions
- Provides shape and stability to cells
- Enables movement, especially in immune cells that must travel to infection sites
- Supports cell division
- Helps cells engulf pathogens
- Facilitates intracellular transport
Why Actin Is Essential
Actin filaments constantly assemble and disassemble, allowing cells to respond quickly to their environment. These filaments form various structures—straight bundles, branched networks, and contractile rings. The Arp2/3 complex, which ARPC5 is part of, specifically builds branched networks essential for shape changes and dynamic movement. Without ARPC5, this branching process collapses.
Cytoskeleton and Immunity
Immune cells rely heavily on actin networks:
- Macrophages need them to swallow bacteria.
- T cells need them to form connections with other immune cells.
- Neutrophils depend on them for rapid movement toward infection sites.
This explains why defects in ARPC5 create such widespread immune dysfunction.
Why the Microbiome Is So Sensitive to Immune Structure
The gut is home to trillions of microbes. Under normal conditions, immune cells maintain a balanced relationship with these microbes—tolerating beneficial ones and containing any harmful ones. When the structural machinery inside immune cells fails, this balance collapses. The microbiome becomes a trigger for inflammation instead of a partner in maintaining health.
This study highlights how delicate that relationship is and how it depends on the physical integrity of immune cells.
Research Reference
Branched actin networks mediate macrophage-dependent host-microbiota homeostasis
https://www.science.org/doi/10.1126/science.adr9571