Why Some Multiple Myeloma Patients Stay Cancer-Free for Years After CAR T Therapy With Cilta-Cel

A new study from the Icahn School of Medicine at Mount Sinai is offering one of the clearest explanations yet for why some people with multiple myeloma remain cancer-free for more than five years after receiving the CAR T therapy ciltacabtagene autoleucel, commonly called cilta-cel. The research, published in Blood Advances, is being described as the first longitudinal, single-cell, multi-omic investigation of how this treatment behaves inside the body over time. It turns out that long-term remission may depend not only on the infused CAR T cells but also on how effectively the patient’s own immune system supports them.
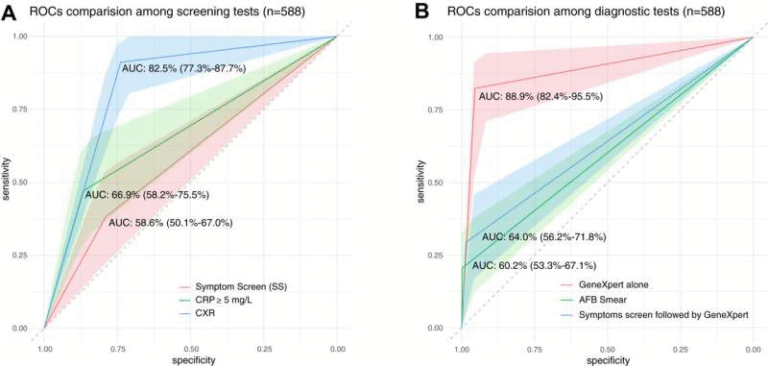
This study looked at 19 patients who received cilta-cel during the CARTITUDE-1 clinical trial. These were patients with relapsed or refractory multiple myeloma, meaning they had tried other treatments that failed to stop the cancer. For each patient, researchers analyzed blood and bone marrow samples both before and after the infusion, tracking how their immune systems behaved across months and years.
One major detail the researchers highlight is that cilta-cel is not just another therapy added to a treatment schedule. It is a one-time infusion made from a patient’s own T cells. Those cells are genetically engineered to hunt down cancer cells carrying BCMA, or B cell maturation antigen, a surface protein commonly found on malignant myeloma cells. Once infused, these modified cells ideally expand quickly, kill off cancer cells, and persist long enough to keep the disease in check.
But not all patients benefit to the same degree. Some go into deep remission that lasts for years, while others relapse much sooner. The Mount Sinai team wanted to figure out what separates the long-term responders from those who see their cancer return early.
Their results suggest that the difference lies in a synergistic interaction between the infused CAR T cells and the patient’s existing immune system. In patients who stayed cancer-free for five years or more, the scientists saw an early and highly focused expansion of CAR T cells soon after treatment. That early expansion is important because it signals that the engineered cells are doing their job effectively and multiplying quickly enough to overwhelm the cancer.
Alongside this early CAR T activity, long-term survivors also displayed a broad and diverse population of healthy CD4 helper T cells. These are the cells that help coordinate the immune response. The fact that they were present in high diversity — and stayed active long after the infusion — seems to strengthen the CAR T cells’ ability to maintain remission. In simpler terms, patients with strong, supportive immune environments gave the CAR T cells room to work and continue working.
The opposite pattern appeared in those who relapsed earlier. These patients tended to have a higher tumor burden at baseline, meaning they had more myeloma cells present before they even received treatment. A heavier disease load is often harder to suppress, even with a technically powerful therapy like cilta-cel. More strikingly, these patients experienced an early surge in immunosuppressive myeloid cells. These myeloid cells can actively weaken T-cell responses, shutting down the very mechanism CAR T therapy relies on. Their early rise essentially blunts the impact of the treatment.
The researchers interpret these findings to mean that long-term remission depends on more than just engineering potent CAR T cells. It requires a balanced immune system that can help sustain them. This insight could help clinicians decide who will benefit most from CAR T therapy, who may need closer monitoring for relapse, and what additional treatments or combinations might strengthen the immune response.
The team plans to validate their findings in larger groups of patients. One goal is to create a simple blood test or biomarker panel that predicts whether a patient is likely to experience long-term remission. If successful, this could significantly improve patient selection and treatment planning.
Since this study is highly specific, it’s also helpful to understand some broader context about multiple myeloma, CAR T therapy, and cilta-cel itself, especially for readers who want to learn more about the science behind the news.
Understanding Multiple Myeloma
Multiple myeloma is a cancer that forms in plasma cells, a type of white blood cell responsible for producing antibodies. When these cells become cancerous, they crowd out healthy blood-forming cells in the bone marrow and produce abnormal proteins that can damage organs such as the kidneys. Symptoms often include bone pain, repeated infections, and fatigue due to anemia.
Myeloma is considered treatable but not yet curable. Over the past two decades, treatments such as immunomodulatory drugs, proteasome inhibitors, monoclonal antibodies, and stem cell transplants have transformed survival rates. However, the disease almost always returns, which is why therapies like CAR T cells have generated major excitement.
Cilta-cel is one of the most advanced treatments available for relapsed or refractory cases. It targets BCMA, a marker very commonly found on myeloma cells, making it ideal for precision immunotherapy.
How CAR T Therapy Works
CAR T cell therapy begins by collecting T cells from the patient. Those cells are then genetically modified in a laboratory to express a chimeric antigen receptor (CAR) that recognizes specific proteins on tumor cells. For cilta-cel, that target is BCMA.
Once the modified T cells are infused into the patient, they seek out cancer cells, bind to BCMA, and kill them. But unlike chemotherapy, which stops working soon after treatment ends, CAR T cells can remain in the body for months or years, continuing to patrol for cancer cells. This durability is what makes the therapy so promising.
However, successful therapy depends on more than just killing tumor cells. CAR T cells must expand effectively, persist long enough to prevent relapse, and function within the immune system without being suppressed. This is exactly where the new study sheds light.
Why This New Research Matters
The new findings from Mount Sinai highlight something that scientists have suspected but not fully documented: CAR T cells don’t act alone. Their effectiveness depends on whether the rest of the immune system supports or interferes with them.
If a patient’s immune system maintains diverse, active helper T cells, and avoids generating too many suppressive myeloid cells, the CAR T cells can persist and remain functional for years. If not, relapse becomes more likely.
This research could influence future clinical decisions in several ways:
- Doctors may begin evaluating immune diversity as part of the selection process for CAR T therapy.
- Biomarkers might help identify patients at high risk of early relapse.
- Treatment strategies might shift toward strengthening the immune environment before or after CAR T infusion.
Given the cost and complexity of CAR T therapy, improving prediction and personalization would be a major step forward for patients and healthcare systems.
Overall, the study adds meaningful clarity about long-term remission after cilta-cel. While the sample size was small (only 19 patients), it provides a solid foundation for future work that could make remission more consistent and more predictable.
Research Reference:
Long-term Remission After Cilta-Cel in Multiple Myeloma Is Linked to Diverse T Cells and Low Myeloid Suppression (Blood Advances, 2025)