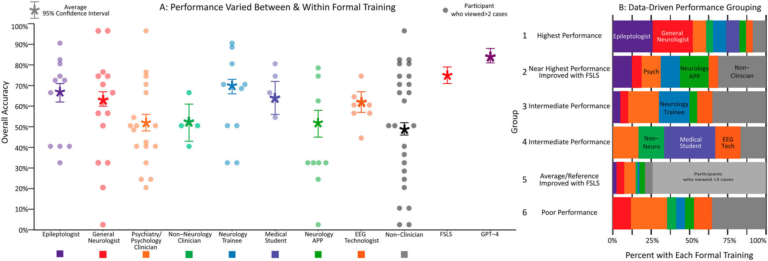
Disrupting Bacterial Communication May Hold the Key to Healthier Teeth and Gums
Researchers from the University of Minnesota have uncovered a fascinating new angle on oral health: instead of killing harmful bacteria, what if we could simply stop them from talking to each other? A newly published study suggests that disrupting bacterial communication—often called quorum sensing—might help shift dental plaque toward a healthier balance and reduce the risk of periodontal disease. The findings offer a fresh perspective on how oral bacteria behave, how dental plaque forms, and how future therapies might work.
Dental plaque forms through a predictable sequence. First come the pioneer species, including Streptococcus and Actinomyces, which typically support good oral health. Over time, other microbes join in, eventually including disease-linked species such as the red-complex bacteria, known for their association with periodontal disease. In this new study, researchers reveal how bacterial communication helps determine which microbes thrive during each stage of plaque development—and how interrupting this communication may reshape the entire community.
The key players in this communication system are molecules called N-acyl homoserine lactones (AHLs). More than 700 bacterial species live in the human mouth, and many use AHLs to send chemical messages that help them coordinate their behavior. These signals influence how bacteria form biofilms, compete for space, and transition into disease-associated communities. What makes this study particularly notable is the discovery that AHL signals are active both above and below the gumline, but they behave very differently depending on oxygen levels.
The research team found that bacteria in dental plaque produce AHLs in oxygen-rich environments like the area above the gums. Surprisingly, microbes in oxygen-poor environments—like the deeper pockets beneath the gums—can still detect these signals even though oxygen is limited. This means bacteria in different regions of the mouth can communicate across ecological boundaries. The team tested whether breaking this communication channel could influence which microbial groups dominate the plaque environment. Using specialized enzymes called lactonases to degrade AHL molecules, they observed a clear shift toward species associated with better oral health.
Under aerobic conditions, removing AHL signals encouraged the growth of beneficial plaque species. But when AHLs were added under anaerobic conditions, the balance tipped in favor of harmful, disease-associated bacteria, particularly the late colonizers that flourish in environments linked to periodontal disease. This dual response suggests that quorum sensing may play very different roles depending on the physical location within the mouth. It also highlights the complexity of designing therapies intended to alter bacterial behavior—one approach may work well in one area but produce the opposite effect elsewhere.
The researchers emphasize that dental plaque functions like a developing ecosystem, similar to a forest that begins with hardy pioneer plants and gradually becomes more diverse. In a healthy mouth, early colonizers maintain stability. But when late-stage bacteria take over—especially those tied to gum disease—problems arise. The study proposes that by interrupting AHL signaling, it may be possible to keep the plaque community in its healthier, early-stage composition and prevent the shift toward disease.
This concept, often referred to as quorum quenching, marks a departure from traditional dental approaches. Instead of using antimicrobials that indiscriminately kill bacteria (including helpful ones), quorum quenching aims to modulate the microbial environment. By adjusting the chemical signals bacteria rely on, researchers hope to encourage a natural, beneficial balance rather than wiping out entire microbial communities.
The implications extend well beyond oral health. Similar communication systems exist throughout the human body, and disruptions in the microbiome are connected to various conditions, including certain cancers, inflammatory diseases, and gastrointestinal disorders. If scientists can learn to selectively modify microbial communication, new therapeutic strategies could eventually emerge for many conditions related to microbiome imbalance.
The next stage of research involves investigating how bacterial signaling varies across different parts of the mouth and across different stages of periodontal disease. Human dental plaque is incredibly diverse, and factors such as oxygen availability, diet, oral hygiene habits, and an individual’s immune response all influence how bacterial communities form and communicate. Understanding these dynamics in real-world conditions will be essential before any therapy can be used in clinics.
The ultimate goal is to create targeted treatments—possibly in the form of mouthwashes, gels, or slow-release dental materials—that help maintain a healthy oral microbiome by strategically suppressing harmful communication pathways. Such therapies could reduce reliance on antibiotics, lower treatment resistance, and treat disease at its earliest stages by stopping harmful microbial shifts before they occur.
To appreciate how promising this approach is, it helps to understand the broader concept of quorum sensing. Bacteria rely on chemical signaling to monitor their population density. When enough bacteria are present to trigger a response, they collectively switch behaviors—such as forming biofilms, producing toxins, or increasing their metabolic activity. This coordinated behavior gives bacterial communities remarkable adaptability. In the case of oral bacteria, quorum sensing helps them organize the layered structure of dental plaque and determine when to transition from harmless community members to disease-associated colonizers.
In many Gram-negative bacteria, AHLs serve as the main signaling molecules. These molecules drift between cells, accumulate in the environment, and bind to receptor proteins to activate specific genes. When researchers break down AHLs using enzymes like lactonases, bacteria can no longer “hear” each other, preventing them from coordinating harmful activities. This idea has been explored in agriculture, wastewater treatment, and medical research, but its application to dental health is still emerging.
The University of Minnesota study brings momentum to this field by demonstrating that quorum quenching can reliably shift the biological makeup of dental plaque under controlled conditions. Beneficial species become more prominent, and harmful species lose their communicative advantage. More importantly, the researchers documented specific differences in how bacteria respond above and below the gumline, a detail that could shape the design of future oral health treatments.
Although more research is needed, the study lays groundwork for an entirely new category of periodontal therapy—one focused on communication disruption instead of microbial destruction. This aligns with modern understandings of the microbiome: health isn’t achieved by sterilizing the body, but by fostering balance. Quorum quenching may allow dentists and physicians to guide that balance more precisely than ever before.
For now, the work represents an exciting step forward in understanding how bacteria in dental plaque interact and how those interactions influence human health. By learning to speak the language of microbes—or silence it—scientists may someday transform how we approach oral disease prevention, not by fighting bacteria, but by redirecting their natural behaviors.
Research Paper:
https://www.nature.com/articles/s41522-025-00846-z