New Immune Cell Mapping Shows How Specific CD4+ T-Cell Subtypes May Drive Lupus in Children
A new study from Weill Cornell Medicine has taken one of the most detailed looks ever at CD4+ T cells in systemic lupus erythematosus (SLE), uncovering very specific immune cell subtypes that seem to influence how the disease develops and why it can become severe, especially in children. The research uses single-cell RNA sequencing (scRNA-seq) to examine hundreds of thousands of immune cells, revealing a complex landscape of helper, regulatory, and even cytotoxic T-cell states that differ sharply between children with lupus and healthy individuals. These findings are important because they may open the door to targeted treatments that avoid the broad immune suppression currently used in lupus care.
Understanding Why This Study Matters
SLE affects more than one million people in the United States, and about 90% of patients are women of childbearing age. Children, however, often experience a more aggressive form of the disease. Many also develop lupus nephritis (LN), a serious kidney complication. Current treatments rely heavily on immunosuppressants, which reduce inflammation but weaken the immune system overall.
CD4+ T cells have long been known to contribute to lupus, but the degree of diversity within this group has not been fully appreciated. The Weill Cornell team wanted to define exactly which T-cell subtypes are driving autoimmunity and tissue damage. Their findings indicate that the cellular landscape is far more detailed than previously recognized.
What the Researchers Actually Did
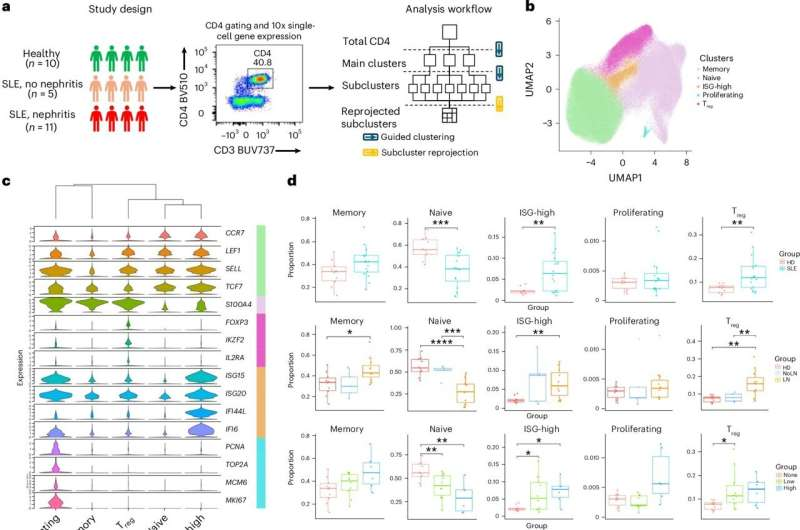
The scientists collected peripheral blood samples from 16 children with SLE (ages 9–17) and 10 healthy controls. Eleven of the patients had biopsy-confirmed lupus nephritis. The team used scRNA-seq to examine 239,473 CD4+ T cells. This huge dataset allowed them to break down the T-cell population into extremely fine-grained subtypes.
They identified five major categories of CD4+ T cells:
- Naive T cells
- Memory T cells
- Regulatory T cells (Tregs)
- Proliferative T cells
- Interferon-stimulated gene–high (ISG-high) T cells
But the most important insights came from the deeper sub-clustering. When they looked more closely, the team uncovered 23 distinct CD4+ T-cell subtypes, each with its own gene-expression signature.
Key Immune Cell Subtypes Expanded in Lupus
Several cell subtypes appeared much more frequently in children with lupus than in healthy controls. These include:
Th10 Cells with Cytotoxic Features
One of the most striking findings was a Th10 subtype that combines features of B-cell help with cytotoxic activity. This is unusual because helper T cells and cytotoxic T cells typically play very different roles.
Even more interesting, the Th10 cells in lupus patients appear to be active outside lymph nodes, in inflamed tissues where they likely provide extra-follicular B-cell help. That kind of environment supports the creation of autoantibodies, the hallmark of lupus.
Because this cell type is both rare and influential, it may be an ideal therapeutic target.
Cytotoxic CD4+ T-Cell Subsets
The study also found an expansion of cytotoxic-like CD4+ T cells, including:
- TEMRA cells (Effector Memory T cells re-expressing CD45RA)
- A subset overlapping with Th10 features
- XCL1+ memory cells, another cytotoxic-leaning population
These subsets were especially common in children with serious disease activity, such as lupus nephritis.
Peripheral Helper T Cells (Tph)
These T cells differ from classical T-follicular helper cells (Tfh). Tph cells do not migrate to lymph nodes. Instead, they operate directly in inflamed tissues, promoting B-cell activation and antibody production. Their expansion in SLE reinforces the idea that lupus involves significant extrafollicular immune activity.
Regulatory T Cells: Abundant but Not Doing Their Job
Regulatory T cells normally keep the immune system under control, preventing excessive inflammation. Surprisingly, the researchers found that Tregs were more abundant in lupus patients—but less functional.
Many of these Tregs displayed pro-inflammatory traits, and even expressed receptors usually associated with mucosal environments, such as:
- TLR5
- FCRL3
These unusual features hint at a possible connection between lupus and microbial dysbiosis, a disturbance in the body’s microbial communities that has been observed in previous studies of SLE.
This dysfunction may help explain why regulatory mechanisms fail to calm autoimmunity in lupus.
What This Means for Future Lupus Treatment
This study shows how incredibly diverse CD4+ T cells are in SLE and how very specific subpopulations may shape disease severity. Instead of relying on broad immunosuppression, future therapeutics might target:
- The Th10 subgroup
- Cytotoxic CD4+ memory subsets
- Dysfunctional Tregs
These narrower targets could reduce symptoms while preserving overall immune function.
The researchers are also exploring whether these specific T-cell subsets can become biomarkers for:
- Monitoring disease severity
- Predicting lupus nephritis
- Detecting early flares
The fact that the team sequenced such a huge number of cells was key. When dealing with a disease as complex as lupus, identifying rare but important subtypes requires exceptionally deep profiling.
A Bit More Background on Lupus and CD4+ T Cells
To make sense of these findings, it helps to understand where CD4+ T cells fit into the bigger picture.
What CD4+ T Cells Do
CD4+ T cells coordinate immune responses. They help other immune cells, including B cells, activate and produce antibodies. Specific subsets can also regulate the immune system or initiate inflammation.
In lupus, CD4+ T cells can become:
- Autoreactive
- Excessively inflammatory
- Poor regulators of other cells
This makes them central players in disease progression.
Why Extra-Follicular B-Cell Help Matters in Lupus
B cells normally mature inside lymph nodes. But in lupus, antibody-producing cells often form outside these structures—especially in inflamed tissues. T-cell subsets like Th10 and Tph may fuel this faulty response.
Why Cytotoxic T Cells Are Unexpected
Cytotoxic activity is usually associated with CD8+ T cells. Seeing this in CD4+ cells, and seeing it so strongly in lupus, suggests an alternative pathway contributing to tissue damage.
Why Dysregulated Tregs Are a Big Deal
If regulatory T cells fail, the immune system loses one of its main braking systems. This aligns with the chronic inflammation and autoantibody production seen in lupus.
Why Children Were the Focus
Childhood-onset lupus tends to be:
- More severe
- More likely to involve the kidneys
- Faster to cause organ damage
Studying pediatric patients gives researchers a clearer view of early immune dysfunction before years of treatment influence cell populations.
A Valuable Resource for Future Studies
The dataset generated—hundreds of thousands of single cells—provides an important reference for the entire lupus research community. Scientists worldwide may use this resource to uncover:
- New immune pathways
- Predictive biomarkers
- Therapeutic targets
The study’s approach also reinforces a key lesson: understanding a complex autoimmune disease requires analyzing large numbers of individual cells to identify rare but influential subtypes.
Reference
Single-cell RNA profiling of blood CD4+ T cells identifies distinct helper and dysfunctional regulatory clusters in children with SLE
https://doi.org/10.1038/s41590-025-02297-2