How the Brain Naturally Protects Itself From Alzheimer’s Disease and Why This Defense Weakens With Age

Alzheimer’s disease is often discussed in terms of memory loss, plaques, and tangles, but long before those hallmarks appear, deeper biological changes are already unfolding inside the brain. A recent study published in JCI Insight sheds light on one such process, revealing how the brain attempts to protect itself from harmful calcium imbalances—and why this protective mechanism gradually fades with age.
At the center of this discovery is a protein called glyoxalase 1 (GLO1), which appears to act as a built-in defense system against cellular stress linked to Alzheimer’s disease. Understanding how this system works could help scientists develop new strategies to prevent or slow neurodegeneration before symptoms become severe.
Why Calcium Balance Matters in the Brain
Calcium is essential for normal brain function. Neurons rely on it to transmit signals, form memories, and adapt to new information. However, too much calcium inside neurons is toxic. When calcium levels rise uncontrollably, it can damage cellular structures, disrupt communication between neurons, and ultimately lead to cell death.
In Alzheimer’s disease, calcium dysregulation has long been suspected as a contributing factor. Excess calcium is known to worsen neuronal loss and cognitive decline, but until now, researchers had limited insight into how the brain initially tries to cope with this problem.
The Role of RyR2 and Calcium “Leakage”
The study focuses on a calcium channel known as ryanodine receptor 2 (RyR2). This channel acts like a faucet, controlling the release of calcium from internal storage compartments within neurons called the smooth endoplasmic reticulum.
Under healthy conditions, RyR2 opens and closes precisely. With aging, however, this channel can become faulty. Instead of opening only when needed, it may remain partially open, causing a chronic calcium leak into the neuron. This constant calcium influx places the cell under stress and has been linked not only to Alzheimer’s disease but also to other neurological conditions, including Long COVID.
GLO1: The Brain’s Hidden Resilience Factor
Researchers from Yale School of Medicine, led by neuroscientist Amy Arnsten and neurologist Lauren Hachmann Sansing, discovered that the brain responds to this calcium overload by increasing levels of GLO1.
GLO1 is a detoxifying enzyme responsible for clearing harmful byproducts produced during normal cellular metabolism. These byproducts, if left unchecked, can damage proteins, lipids, and DNA. When calcium levels rise excessively, the production of these toxic compounds increases, making GLO1 especially important.
The study found that in brains experiencing chronic calcium leakage, GLO1 expression and activity increased significantly, particularly in regions critical for thinking and memory, such as the prefrontal cortex and the hippocampus.
Evidence From Animal Models
To explore this mechanism, researchers used animal models in which RyR2 channels were genetically altered to remain permanently “on.” This created a condition of sustained calcium leakage, mimicking what happens in aging and Alzheimer’s disease.
In these models, GLO1 levels initially rose as the animals aged. In mice, GLO1 expression peaked around 12 months of age, suggesting that the brain actively ramps up its defenses in response to long-term calcium stress.
However, this protective response did not last indefinitely. As the animals grew older, GLO1 activity declined, even though calcium dysregulation persisted. This decline appeared to leave neurons more vulnerable to damage.
Memory Decline Linked to Loss of GLO1 Protection
The researchers then tested the cognitive impact of this change. Older animals were placed in a T-shaped maze, a standard test used to assess memory and learning. Animals with defective RyR2 channels and reduced GLO1 levels performed significantly worse than healthy controls.
This finding provided direct evidence that calcium dysfunction combined with reduced GLO1 protection is associated with impaired cognition. In other words, when the brain can no longer maintain its natural detoxifying response, memory and learning begin to suffer.
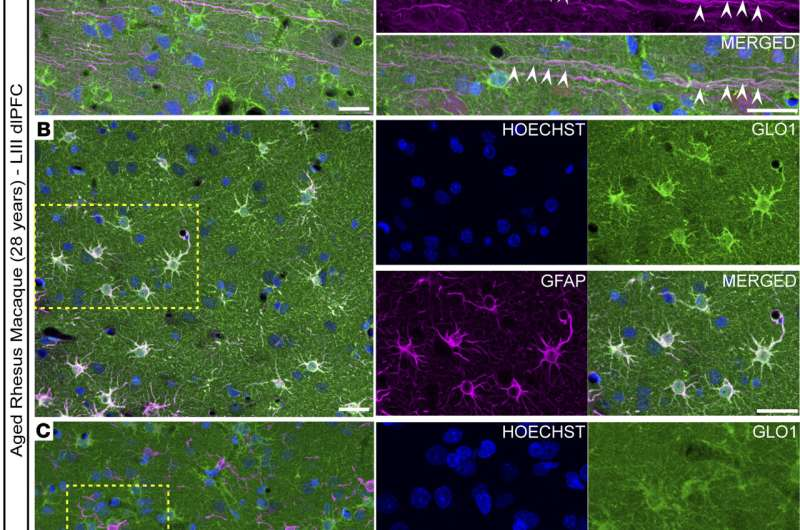
Where GLO1 Is Found in the Brain
Using advanced imaging techniques, the team observed where GLO1 was located within the brain. They found the protein inside excitatory neurons, particularly within dendrites, as well as in astrocytes, which support and regulate neuronal function. GLO1 was also present alongside microglial cells, the brain’s immune defenders.
This widespread distribution suggests that GLO1 plays a broad protective role across multiple brain cell types, not just neurons alone.
Why This Matters for Alzheimer’s Prevention
Most Alzheimer’s treatments focus on the disease after it has already taken hold, targeting amyloid plaques or tau tangles. This research takes a different angle by examining what happens before major degeneration occurs.
The findings suggest that boosting or preserving GLO1 activity could help maintain the brain’s resilience against calcium-related stress. Instead of fighting Alzheimer’s after symptoms appear, future therapies might aim to support the brain’s own protective systems early on, potentially delaying or preventing the disease altogether.
Additional Insight: Calcium Dysregulation in Neurodegenerative Diseases
Calcium imbalance is not unique to Alzheimer’s disease. Similar disruptions have been observed in Parkinson’s disease, Huntington’s disease, and other neurodegenerative conditions. Calcium overload can trigger oxidative stress, mitochondrial dysfunction, and inflammation—all common features of aging brains.
By studying proteins like GLO1, scientists are beginning to understand how cells naturally counteract these stresses and why those defenses weaken over time.
Additional Insight: Glyoxalase Pathways and Brain Health
The glyoxalase system, which includes GLO1, is responsible for neutralizing reactive carbonyl compounds that can form advanced glycation end products (AGEs). AGEs accumulate with age and have been linked to inflammation, vascular damage, and neurodegeneration.
Maintaining efficient glyoxalase activity may therefore be important not only for Alzheimer’s disease but also for overall healthy brain aging.
A Step Toward Preventative Therapies
This study highlights a crucial shift in Alzheimer’s research: focusing on resilience rather than damage. By understanding how the brain naturally protects itself—and why those protections fade—scientists may uncover new therapeutic targets that work alongside the brain instead of against it.
While more research is needed to translate these findings into treatments for humans, the discovery of GLO1’s role provides a promising new direction in the effort to combat Alzheimer’s disease at its earliest stages.
Research paper:
https://insight.jci.org/articles/view/184041