Single-Dose Gene Therapy Shows Real Movement Gains in Older Children and Teens With Spinal Muscular Atrophy
A major new clinical trial has delivered encouraging news for families living with spinal muscular atrophy (SMA), especially those with children who are no longer infants. Researchers have found that a one-time gene replacement therapy can significantly improve movement and motor function in children over 2 years of age and adolescents, a group that until now had very limited treatment options beyond lifelong, repeat-dose therapies.
The findings come from a phase 3 randomized clinical trial published in Nature Medicine (2025) and led by researchers at St. Jude Children’s Research Hospital, including neurologist Richard Finkel. The study focused on onasemnogene abeparvovec, a gene therapy designed to restore production of a missing protein that is essential for motor neuron health.
Understanding Spinal Muscular Atrophy and Why Age Matters
Spinal muscular atrophy is a rare, inherited neuromuscular disorder that causes progressive muscle weakness and loss of movement. The condition occurs because the body cannot produce enough of a critical protein called survival motor neuron (SMN), which is necessary for maintaining healthy motor nerve cells. Without enough SMN protein, these nerve cells deteriorate, leading to worsening muscle function over time.
For years, gene therapy for SMA has been limited by age and weight restrictions. While onasemnogene abeparvovec is already approved in the U.S. and Europe, it has only been authorized as a single intravenous treatment for children under 2 years of age. Older children and teens with SMA have had to rely on treatments that slow disease progression rather than correct the underlying genetic cause, and these treatments require regular injections or daily oral dosing for life.
This new study directly addresses that gap.
How This Gene Therapy Works
Onasemnogene abeparvovec is a gene replacement therapy that uses a viral vector to deliver a functional copy of the SMN1 gene into the body. Once delivered, the gene enables cells to start producing the SMN protein that is missing in people with SMA.
What makes this trial especially important is how the therapy was delivered. Instead of an intravenous infusion, researchers administered the treatment directly into the spinal fluid, a method known as intrathecal delivery. This approach is particularly suited for older and heavier patients, for whom intravenous delivery is not feasible at current approved doses.
Crucially, the therapy is designed as a single-dose treatment, raising the possibility of long-term benefits without the burden of repeated medical procedures.
Inside the Phase 3 Clinical Trial
The trial followed 126 children and adolescents between 2 and 18 years of age. All participants had SMA and shared an important characteristic: they were able to sit independently but had never walked on their own, reflecting a moderate but significant level of disability.
Participants were randomly assigned into two groups:
- 75 participants received the active gene therapy
- 51 participants received a placebo
Neither the participants nor the investigators knew who received which treatment during the study, ensuring a rigorous and unbiased comparison.
The study lasted 12 months, during which researchers closely monitored motor function, safety, and side effects.
Measurable Improvements in Movement and Motor Skills
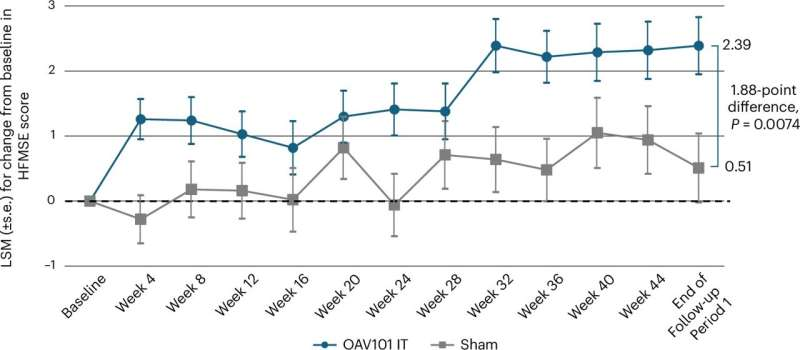
The results were clear and statistically significant. Children and adolescents who received the gene therapy showed greater improvement in motor function compared with those who received placebo.
Motor function was measured using a validated clinical test that evaluates 33 specific motor skills, including:
- Moving from a lying position into sitting
- Standing with support
- Walking-related movements
- Climbing stairs
- Overall control and coordination of muscle groups
On average, participants in the treatment group experienced meaningful gains from baseline in their scores, while improvements in the placebo group were far smaller. These gains translated into real-world functional changes, not just numbers on a scale.
For families and clinicians, this is especially important because SMA treatments often aim only to stabilize decline. Demonstrating actual improvement in older children is a major step forward.
Safety Profile and Side Effects
Safety was a key focus of the trial, particularly given concerns that can accompany gene therapies. The good news is that side effects were generally manageable and similar between the treatment and placebo groups.
No unexpected safety signals emerged during the year-long study. Researchers noted that while careful monitoring is still essential, the findings support the overall safety of intrathecal onasemnogene abeparvovec in this older age group.
That said, the authors emphasized an important caveat: long-term safety and durability of benefit cannot be fully assessed in a 12-month trial. Continued follow-up will be necessary to understand how long the improvements last and whether any delayed effects appear over time.
Why This Study Is Such a Big Deal
This trial represents a potential shift in how SMA is treated beyond infancy. Until now, older children and teenagers with SMA have largely been excluded from gene replacement strategies, despite continuing to experience disease progression.
The findings suggest that gene therapy can still be effective even after early childhood, provided it is delivered in a way that targets the nervous system effectively. This could eventually lead to broader regulatory approvals, giving families access to a one-time treatment instead of lifelong medication schedules.
For many patients, that difference is not just medical but deeply practical, affecting quality of life, treatment burden, and long-term care planning.
How This Fits Into the Broader SMA Treatment Landscape
Currently approved SMA treatments include therapies that:
- Increase production of SMN protein from backup genes
- Slow or stabilize disease progression
- Require repeated administration, sometimes indefinitely
While these treatments have transformed SMA care over the past decade, they do not replace the missing SMN1 gene itself. Gene replacement therapy directly addresses the root cause of the disease, making it a particularly attractive option if proven safe and durable.
This study adds to growing evidence that timing matters, but opportunity remains even beyond early infancy.
What Comes Next
The researchers are clear that this is not the final word. Longer-term studies are needed to:
- Confirm durability of motor improvements
- Monitor long-term safety
- Understand how gene therapy interacts with other SMA treatments
- Determine whether earlier or later intervention within this age range leads to different outcomes
Still, the data strongly support continued development and potential expansion of gene therapy options for people living with SMA.
For families who have long been told that gene therapy was “too late” for their child, this research offers real, data-backed optimism.
Research Reference:
Crystal M. Proud et al., Intrathecal onasemnogene abeparvovec in treatment-naive patients with spinal muscular atrophy: a phase 3, randomized controlled trial, Nature Medicine (2025)
https://doi.org/10.1038/s41591-025-04103-w