Platelet-Inspired Nanoparticles Could Dramatically Improve Brain-Computer Interface Electrodes
Researchers working at the intersection of neuroscience, bioengineering, and nanomedicine have reported a promising advance that could help brain-computer interfaces (BCIs work better and last longer. A team from Case Western Reserve University, collaborating with biotech startup Haima Therapeutics, has shown that platelet-inspired nanoparticles can deliver anti-inflammatory drugs directly to implanted brain electrodes, significantly improving their performance. The study was published in Nature Communications in 2025 and offers a new strategy for tackling one of the biggest challenges facing BCI technology: inflammation in the brain.
Brain-computer interfaces are systems that allow people to control external devices using signals from their brain. They hold enormous promise for people with spinal cord injuries, neurological disorders, or prosthetic limbs, enabling them to move robotic arms, type on a computer, or interact with their environment simply by thinking. However, despite impressive progress, BCIs still struggle with a major biological problem that limits their long-term reliability.
Why Brain Implants Struggle Over Time
When electrodes are implanted into the brain, the procedure inevitably causes vascular injury and disrupts the blood-brain barrier. The brain interprets the electrode as a foreign object, similar to a splinter, and responds with an immune reaction. This response involves inflammation, activation of immune cells such as microglia and astrocytes, and the formation of scar-like tissue around the electrode.
Over time, this inflammatory environment degrades the electrode’s ability to reliably record neural signals. Signals become noisier, fewer neurons can be detected, and eventually the electrode may fail altogether. This biological reaction is one of the main obstacles preventing BCIs from being used reliably over long periods in real-world clinical settings.
Traditionally, researchers have tried to control inflammation using systemic anti-inflammatory drugs, meaning drugs delivered throughout the body. Unfortunately, this approach often fails to protect the electrode site effectively and can cause unwanted side effects elsewhere in the body. In some cases, systemic dosing has even been shown to make electrode performance worse.
Using Platelet Biology as a Targeting Strategy
The new approach takes advantage of a simple but powerful biological insight: platelets naturally accumulate at sites of vascular injury. Platelets are best known for their role in blood clotting, but they also actively home in on damaged blood vessels and areas where the blood-brain barrier has been compromised.
The research team developed platelet-inspired nanoparticles that mimic key features of real platelets. These nanoparticles are designed to circulate through the bloodstream and selectively gather at sites where injury has occurred, including the areas around implanted brain electrodes.
By loading these particles with the anti-inflammatory drug dexamethasone, the researchers created a delivery system that acts like a Trojan horse. Instead of flooding the entire body with medication, the drug is carried directly to the exact place where it is needed most.
How the Study Was Designed
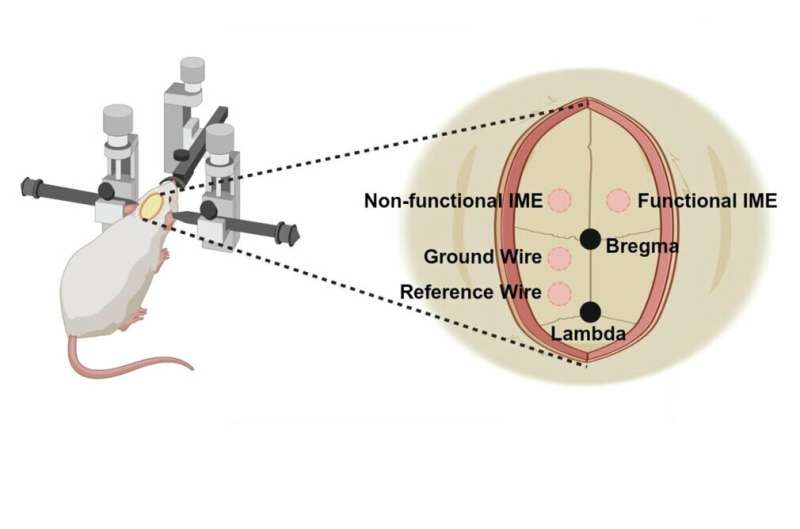
The experiments were conducted using intracortical microelectrodes implanted into the brains of animal models. The researchers performed craniotomies and implanted different types of electrodes, including both functional and non-functional implants, along with bone screws for reference and ground wires. Non-functional implants were included to increase sample size for detailed histological analysis.
The study followed an eight-week timeline. Blood samples were collected, surgeries were performed, and animals received weekly doses of the drug-loaded nanoparticles. Neural recordings were taken every two weeks, and at the end of the study the animals underwent cardiac perfusion and brain tissue collection for detailed examination.
Importantly, the researchers compared three conditions: electrodes with targeted nanoparticle delivery, electrodes with systemic drug delivery, and untreated controls.
Clear Improvements in Electrode Performance
The results were striking. Electrodes treated with dexamethasone-loaded platelet-inspired nanoparticles showed roughly double the recording performance compared with untreated electrodes. These electrodes maintained stronger and more stable neural signals throughout the study period.
In contrast, animals that received the same drug systemically, without targeted delivery, actually showed poorer electrode performance. This finding highlights how critical precise targeting is when dealing with the brain’s delicate immune environment.
Histological analysis of the brain tissue revealed several important changes near the treated electrodes. There was reduced activation of microglia and astrocytes, indicating lower inflammation. The researchers also observed higher neuronal density close to the electrode surface and signs of improved blood-brain barrier integrity.
Together, these findings suggest that the nanoparticles not only delivered the drug effectively but also helped stabilize the local vascular environment around the implant.
The Technology Behind the Nanoparticles
The platelet-inspired nanoparticle platform was developed by Anirban Sen Gupta, a biomedical engineering professor at Case Western Reserve University. The technology has been patented and licensed to Haima Therapeutics, a startup company co-founded by Sen Gupta and alumna Christa Pawlowski, who serves as the company’s chief operating officer.
Haima’s platform, often referred to as SynthoPlate, is designed as a flexible system rather than a single-use product. The particles can be loaded with different drugs depending on the disease or injury being targeted, as long as platelets naturally accumulate at that site.
For this study, Haima Therapeutics manufactured the drug-containing nanoparticles used in the experiments.
What This Means for the Future of BCIs
This research represents an important step toward making BCIs more reliable and clinically viable. By addressing inflammation directly at the implant site, the approach could extend the functional lifespan of brain electrodes and improve signal quality for patients who depend on these systems.
The researchers plan to move the technology toward clinical translation, starting with safety studies. Haima Therapeutics has announced plans to begin human clinical trials around 2027, pending regulatory approval and further preclinical validation.
Broader Applications Beyond Brain Implants
While BCIs are a high-profile application, the implications of this technology go far beyond neural interfaces. Because the nanoparticle platform can target any site of vascular injury, it has potential applications in a wide range of diseases.
Possible future uses include treating stroke, heart attack, autoimmune disorders such as rheumatoid arthritis, and infectious diseases like sepsis. In all of these conditions, inflammation and vascular damage play a central role, making targeted platelet-inspired delivery especially attractive.
This platform approach also opens the door to combining drugs or adjusting dosing strategies in ways that are difficult or impossible with conventional therapies.
A Step Toward Smarter Drug Delivery in the Brain
The study highlights a broader shift in biomedical research toward precision drug delivery. Instead of treating the entire body and hoping the drug reaches the right place, scientists are increasingly designing systems that work with the body’s own biology to deliver therapies exactly where they are needed.
By mimicking platelets, these nanoparticles turn a natural injury response into a therapeutic advantage. For brain-computer interfaces, where even small improvements in signal quality can have a big impact on patient outcomes, this approach could make a meaningful difference.
As BCIs continue to advance and move closer to widespread clinical use, solutions like this one may be essential for overcoming the biological challenges that come with long-term brain implantation.
Research paper:
https://www.nature.com/articles/s41467-025-63583-z