Bioluminescent Brain Imaging Tool CaBLAM Lets Scientists Watch Neural Activity Without External Lasers
A team of neuroscientists has developed a powerful new brain-imaging tool that allows researchers to observe neural activity using bioluminescence instead of external light sources like lasers. The technology, known as CaBLAM (Calcium BioLuminescence Activity Monitor), marks a major step forward in how scientists can safely, clearly, and continuously study living brain cells in action.
The work comes from researchers connected to the Bioluminescence Hub at Brown University’s Carney Institute for Brain Science, working alongside collaborators from several other institutions. Their findings were published in the journal Nature Methods in December 2025.
What CaBLAM Is and Why It Matters
CaBLAM is a genetically encoded calcium indicator that produces its own light when neurons become active. Because calcium ions play a central role in neural signaling, tracking calcium fluctuations is one of the most reliable ways to measure brain activity. Until now, most calcium-imaging techniques relied on fluorescence, which requires shining powerful external light into the brain.
That traditional approach works, but it comes with serious drawbacks. Strong illumination can damage cells, degrade fluorescent molecules over time through photobleaching, and require invasive hardware such as lasers, fiber optics, and implanted lenses. CaBLAM avoids all of these issues by allowing neurons to generate light from within, eliminating the need for external illumination entirely.
How Bioluminescence Changes Brain Imaging
Bioluminescence occurs when an enzyme chemically reacts with a small molecule to produce light. In CaBLAM’s case, this reaction has been engineered so that light output directly reflects calcium levels inside the cell. When a neuron becomes active and calcium concentrations rise, CaBLAM glows brighter.
This internal light production offers several advantages:
- No phototoxicity, because no external light is used
- No photobleaching, allowing recordings to last much longer
- Minimal background noise, since brain tissue does not naturally emit bioluminescent light
- Clearer signals deep inside the brain, where external light often scatters and weakens
Because the signal comes only from active cells, neurons essentially act as their own biological headlights, making activity easier to detect even through dense tissue.
Capturing Brain Activity in Real Time
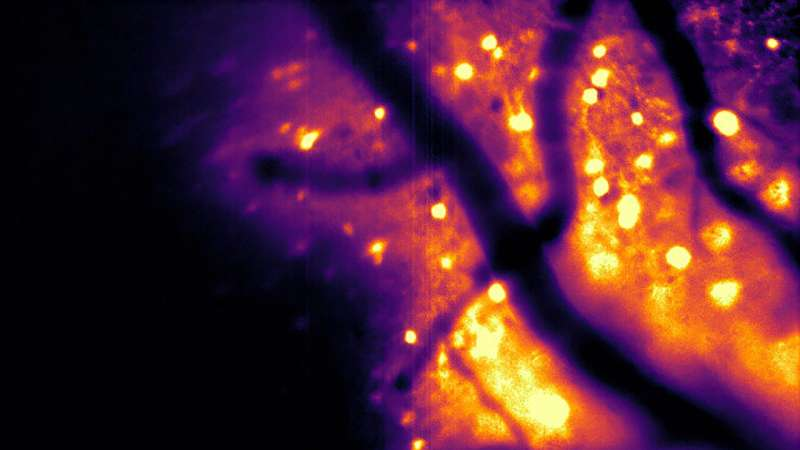
In laboratory tests, CaBLAM demonstrated the ability to capture single-cell and even subcellular activity at high speeds. Researchers successfully recorded neurons firing in the brains of live mice while the animals were awake and moving, including while running on a wheel.
One of the most striking achievements was the duration of the recordings. Using CaBLAM, scientists recorded continuous neural activity for five hours, something that would be extremely difficult or impossible with fluorescent imaging due to photobleaching and tissue stress.
The tool also performed well in zebrafish models, showing its versatility across species. This opens the door to studying brain activity over long periods during complex behaviors such as learning, decision-making, and motor coordination.
Who Developed CaBLAM
The Bioluminescence Hub launched in 2017 with the goal of creating and sharing tools that allow neurons to both produce and respond to light. Key contributors to this effort include researchers from Brown University, Central Michigan University, the University of California San Diego, UCLA, and New York University.
The molecular foundation of CaBLAM was developed by a neuroscientist at UC San Diego, who engineered the sensor from a luciferase enzyme originally derived from the deep-sea shrimp Oplophorus gracilirostris. This enzyme was modified to dramatically increase brightness and sensitivity, solving a long-standing challenge in bioluminescent imaging.
For decades, scientists had explored the idea of using bioluminescence to monitor brain activity, but the emitted light was simply too dim for detailed imaging. CaBLAM is the first tool to overcome that limitation at a level suitable for high-resolution neuroscience research.
Advantages Over Fluorescent Calcium Imaging
Fluorescent calcium indicators remain widely used, but they have inherent limitations:
- They require constant illumination, which can damage tissue
- Fluorescent molecules lose effectiveness over time
- External light causes autofluorescence, adding visual noise
- Imaging equipment tends to be bulky and invasive
CaBLAM sidesteps these problems entirely. Because the brain does not naturally glow, bioluminescent neurons appear against a dark, high-contrast background, producing cleaner data with less interference.
This simplicity also reduces the amount of hardware needed, making experiments easier to set up and potentially less stressful for animal subjects.
Beyond Imaging: A Broader Vision for Light-Based Neuroscience
CaBLAM is part of a larger effort to rethink how light can be used in neuroscience. Researchers at the Bioluminescence Hub are also exploring ways for neurons to communicate with each other using light, effectively creating new signaling pathways inside the brain.
Other projects focus on using calcium itself not just as a readout of activity, but as a control mechanism for cellular behavior. These ideas all depend on having brighter, more reliable calcium sensors, making CaBLAM a foundational piece of a much larger toolkit.
Potential Applications Outside the Brain
Although CaBLAM was developed with neuroscience in mind, its usefulness may extend far beyond the brain. Calcium signaling is critical in many parts of the body, including:
- Heart muscle contraction
- Immune cell activation
- Hormone secretion
- Muscle movement
Because CaBLAM allows long-term, low-stress imaging, it could eventually be adapted to study multiple organs at once, providing insights into how different systems communicate in real time.
Why This Research Stands Out
More than 34 researchers contributed to the development and testing of CaBLAM, making it a clear example of collaborative, cross-institutional science. The tool not only solves technical problems but also changes what kinds of questions scientists can realistically ask.
By enabling long-duration, high-contrast imaging without damaging tissue, CaBLAM allows researchers to observe biological processes as they unfold naturally, rather than in short, interrupted snapshots.
This approach could reshape studies of learning, memory, disease progression, and whole-body physiology in the years ahead.
Research Paper Reference
Lambert, G. G. et al. (2025). CaBLAM: a high-contrast bioluminescent Ca²⁺ indicator derived from an engineered Oplophorus gracilirostris luciferase. Nature Methods.
https://doi.org/10.1038/s41592-025-02972-0