New Research Reveals How the GDF3 Protein Keeps Inflammation Switched On in Aging Immune Systems
As people grow older, their immune systems don’t just weaken—they often become misguided. Instead of simply defending the body against infections, immune cells can remain stuck in a constant state of low-grade inflammation. This chronic inflammation, often called inflammaging, has long been linked to age-related diseases and higher vulnerability to severe infections like sepsis. A new study published in Nature Aging now sheds light on a key molecular driver behind this problem: a protein called GDF3.
Researchers from the University of Minnesota have identified how GDF3 helps lock certain immune cells into a persistent inflammatory mode as organisms age. The findings provide a detailed explanation of why inflammation becomes harder to turn off in older adults—and why this can have serious consequences during infections.
Understanding the Role of Macrophages in Aging
At the center of this research are macrophages, a type of immune cell that plays a major role in inflammation. Macrophages are essential for detecting pathogens, clearing damaged tissue, and coordinating immune responses. However, in aging bodies, these cells often behave differently.
Instead of responding to threats and then calming down, aging macrophages—especially those found in adipose (fat) tissue—tend to remain in a chronically activated inflammatory state. This ongoing inflammation can damage surrounding tissues and organs and can dramatically worsen outcomes during infections.
The new study focused on understanding why macrophages fail to shut down inflammation with age, and what molecular signals are responsible.
GDF3 Emerges as a Key Driver of Chronic Inflammation
The researchers discovered that aging macrophages produce increased levels of Growth Differentiation Factor 3 (GDF3), a signaling protein that belongs to the larger TGF-β superfamily. What makes GDF3 especially important is that it works through an autocrine feedback loop—meaning the macrophages release GDF3, and then that same protein signals back to the very cells that produced it.
This feedback loop reinforces inflammatory behavior instead of resolving it. In simple terms, macrophages are telling themselves to stay inflamed, even when doing so becomes harmful.
How GDF3 Rewires Macrophages at the Genetic Level
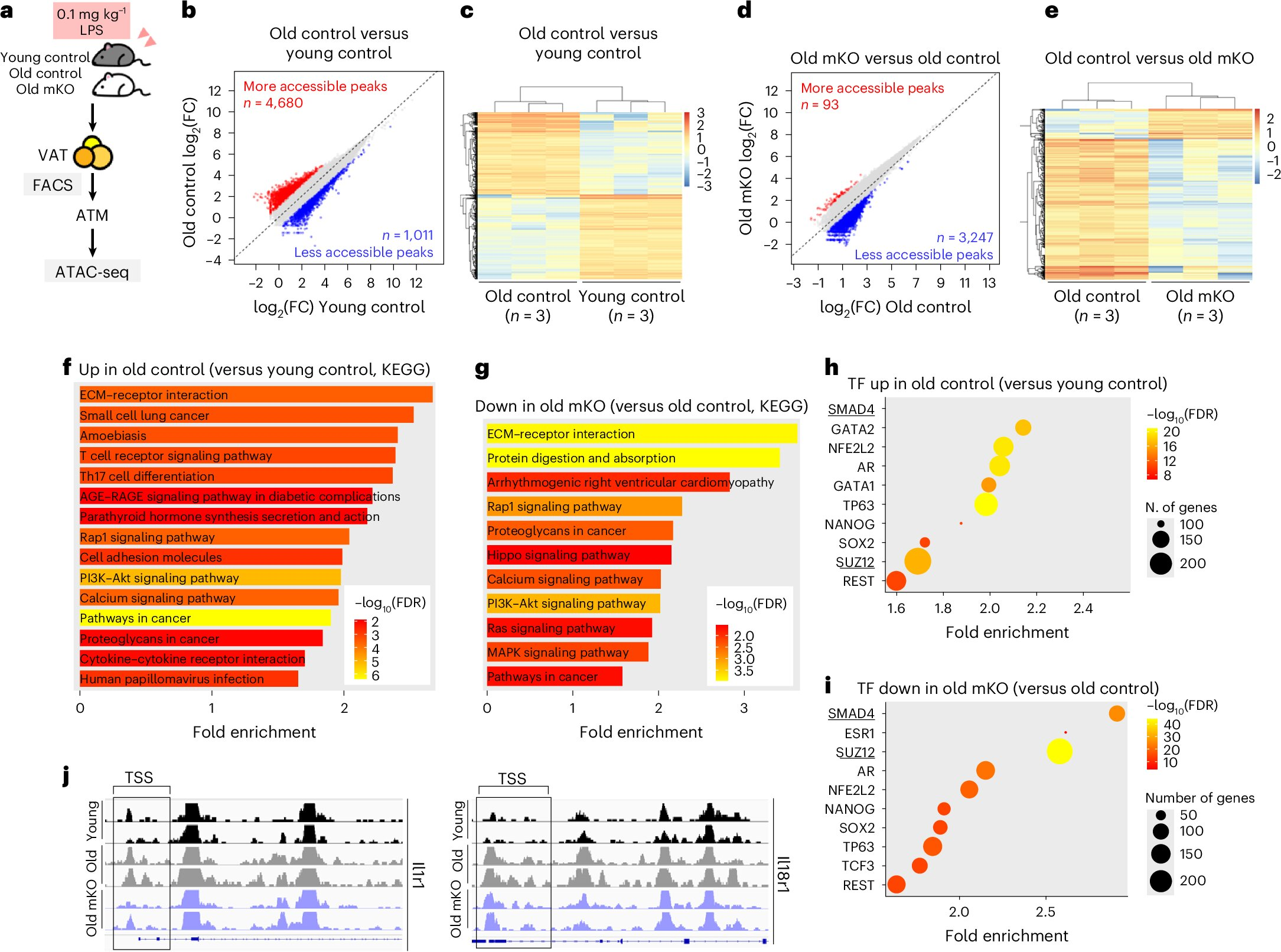
One of the most significant findings of the study is how GDF3 changes macrophage behavior at a genomic and epigenetic level. GDF3 activates a signaling pathway involving SMAD2 and SMAD3, which are transcriptional regulators inside the cell.
When SMAD2/3 signaling is activated by GDF3, it doesn’t just temporarily turn genes on or off. Instead, it alters chromatin accessibility—the way DNA is packaged inside the cell. These chromatin changes make pro-inflammatory genes easier to access and more likely to stay active over time.
As a result, macrophages begin producing higher levels of inflammatory cytokines on a long-term basis. This effectively locks the cells into a chronic inflammatory identity, even in the absence of immediate threats.
Evidence from Preclinical Aging Models
To test the real-world impact of GDF3, the researchers used preclinical aging models, including older mice exposed to bacterial toxins that mimic severe infection and sepsis.
The results were striking. Older animals with intact GDF3 signaling showed:
- Exaggerated inflammatory responses
- Poorer regulation of immune activity
- Worse survival outcomes following severe infection
When the GDF3 gene was removed, these harmful responses were significantly reduced. Macrophages produced fewer inflammatory molecules, and the animals showed improved resilience when challenged with infection-related stress.
Blocking the GDF3–SMAD Pathway Improves Outcomes
Beyond genetic deletion, the researchers also tested pharmacological approaches. Drugs that block the GDF3–SMAD2/3 signaling pathway were able to alter the behavior of inflammatory macrophages in fat tissue.
These interventions reduced harmful inflammation and improved survival in older preclinical models exposed to severe infections. This suggests that the pathway is not only important biologically, but also potentially druggable, opening the door to future therapies.
Links to Human Aging and Inflammation
To see whether these findings were relevant to humans, the research team collaborated with scientists from the School of Public Health and analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study, a long-running population study.
They found that circulating GDF3 protein levels in older adults correlated strongly with inflammatory signaling markers. While this part of the research does not prove causation, it provides compelling evidence that the same mechanisms observed in preclinical models may also operate in aging humans.
Why Chronic Inflammation Is So Dangerous in Older Adults
Persistent inflammation is not just an abstract biological concept—it has very real consequences. Chronic inflammatory signaling contributes to:
- Increased risk of sepsis
- Poorer recovery from infections
- Damage to vital organs
- Higher susceptibility to age-related diseases
In sepsis specifically, an overactive inflammatory response can be more dangerous than the infection itself. The study helps explain why older adults often experience more severe and deadly inflammatory reactions, even to infections that younger immune systems handle more effectively.
The Broader Context of Inflammaging
The concept of inflammaging has been discussed for decades, but identifying precise molecular drivers has been challenging. This research places GDF3 firmly on the list of proteins that may actively maintain chronic inflammation with age.
By showing how GDF3 reshapes chromatin and locks macrophages into an inflammatory state, the study adds depth to our understanding of how aging affects immune regulation at the most fundamental levels.
What Comes Next for GDF3 Research
The authors emphasize that more research is needed before any clinical treatments become available. Key next steps include:
- Identifying additional molecular partners involved in GDF3 signaling
- Understanding how this pathway interacts with metabolism and fat tissue
- Determining whether targeting GDF3 can reduce inflammation without impairing normal immune defense
- Exploring GDF3 as a potential biomarker for age-related inflammatory risk
If future studies confirm these findings in clinical settings, targeting the GDF3–SMAD pathway could become a promising strategy for reducing harmful inflammation in older adults.