Scientists Discover RNA Quality Control Failure Linked to ALS Progression
A new study from Northwestern Medicine has uncovered a crucial molecular breakdown that helps explain why motor neurons deteriorate in amyotrophic lateral sclerosis (ALS). The research shows that a fundamental cellular safeguard—an RNA quality control system designed to prevent faulty genetic messages from causing damage—fails in ALS, allowing harmful RNA molecules to build up inside neurons.
The findings were published in the journal Neuron and provide fresh insight into how disruptions in RNA metabolism contribute directly to neurodegeneration. At the center of this discovery are two proteins, TDP-43 and UPF1, whose normal partnership is essential for keeping RNA messages accurate, stable, and safe for cells to use.
ALS and the Importance of RNA Quality Control
Every cell in the human body relies on RNA as a messenger that carries instructions from DNA to produce proteins. These instructions must be precise. If RNA messages are incomplete, incorrectly processed, or damaged, the resulting proteins can malfunction or become toxic.
To prevent this, cells use a sophisticated surveillance process known as mRNA decay, which identifies defective RNA molecules and destroys them before they can do harm. One of the most important players in this system is UPF1, a protein that acts like a molecular proofreader, scanning RNA transcripts and triggering the removal of faulty ones.
In healthy neurons, this system runs quietly in the background. But in ALS, the Northwestern Medicine team found that this RNA cleanup process is severely compromised.
The Central Role of TDP-43 in ALS
Most ALS cases share a common pathological feature involving TDP-43, an RNA-binding protein that normally resides in the cell nucleus. Under normal conditions, TDP-43 helps regulate RNA processing, stability, and transport.
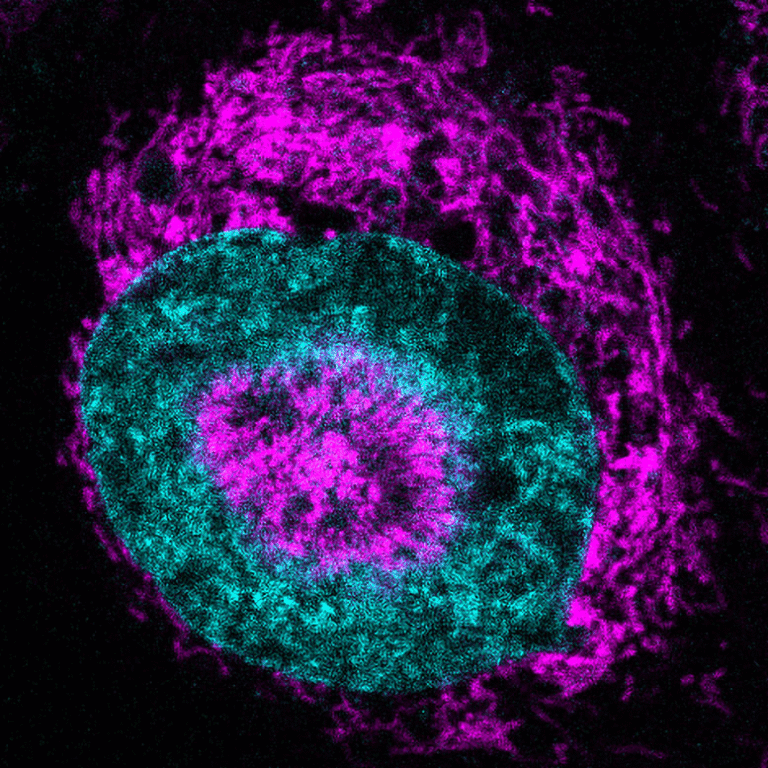
In ALS, however, TDP-43 leaves the nucleus and accumulates in the cytoplasm, where it forms clumps known as aggregates. This mislocalization leads to a loss of normal TDP-43 function, which is widely considered a major driver of ALS pathology.
The new study focused on understanding how the loss of TDP-43 function affects RNA metabolism in human motor neurons, particularly its relationship with UPF1.
How UPF1 and TDP-43 Work Together
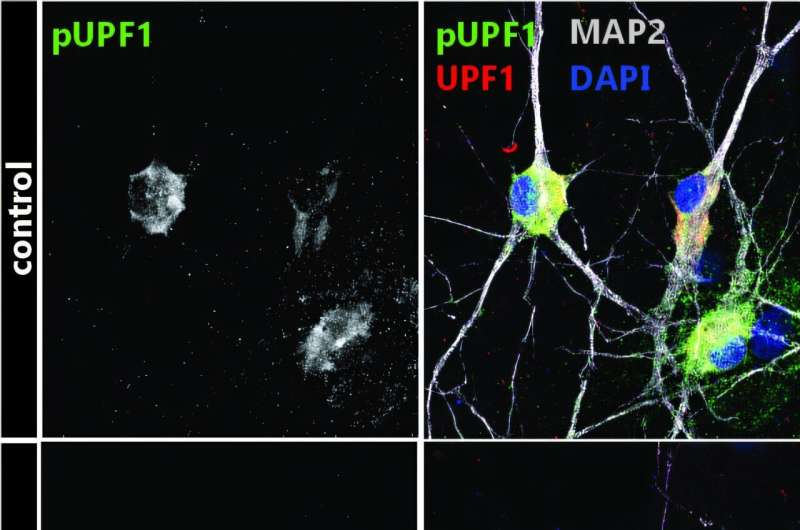
Using motor neurons derived from induced pluripotent stem cells (iPSCs), the researchers examined how UPF1 behaves when TDP-43 is depleted. What they found was striking.
Under normal conditions, TDP-43 and UPF1 interact closely, forming an RNA-dependent partnership that helps regulate the length and structure of RNA molecules, especially at their tail ends, known as 3′ untranslated regions (3′ UTRs). These regions play a key role in determining how long an RNA molecule survives and where it travels inside the cell.
When TDP-43 function is lost, UPF1 activity drops significantly. The study showed that motor neurons from ALS patients—and neurons experimentally depleted of TDP-43—had much lower levels of active UPF1. As a result, defective RNAs that should normally be destroyed are allowed to persist.
Adding to the problem, the researchers observed that UPF1 and TDP-43 physically clump together in ALS-affected neuronal tissue, further disrupting their normal roles.
A Double Hit to Neuronal Health
The breakdown of this system represents what researchers describe as a double failure. First, TDP-43 is no longer able to perform its regulatory duties. Second, UPF1 loses its ability to efficiently eliminate defective RNA molecules.
This allows abnormal RNAs—many of which arise as a direct consequence of TDP-43 dysfunction—to escape degradation and accumulate inside motor neurons. Over time, this buildup places enormous stress on the cells, making them more vulnerable to degeneration.
The study identified that many of the RNAs affected by this breakdown contain long, GC-rich 3′ UTRs, which are particularly dependent on UPF1-mediated surveillance. When this system fails, these unstable RNAs linger far longer than they should.
What This Means for ALS Research
This research connects several important dots in ALS biology. While scientists have long known that TDP-43 mislocalization is a hallmark of ALS, this study provides clear evidence that its loss directly undermines RNA quality control by impairing UPF1 function.
By mapping the RNAs that accumulate when UPF1 activity is reduced, the researchers now have a clearer picture of which genetic messages may be contributing to motor neuron toxicity. Understanding exactly how these RNAs damage neurons is a major next step.
Potential Therapeutic Implications
One of the most promising aspects of this discovery is its therapeutic potential. If defective RNA accumulation is a key driver of neuron loss, then restoring UPF1 activity or reactivating the RNA decay pathway could help slow or even halt disease progression.
Rather than targeting symptoms, this approach aims to correct a core molecular failure within ALS neurons. While translating these findings into treatments will take time, the study opens new directions for drug development focused on RNA metabolism and surveillance.
Why RNA Metabolism Matters in Neurodegenerative Diseases
ALS is not the only neurological disorder linked to RNA dysfunction. Increasing evidence suggests that RNA processing errors play a role in several neurodegenerative diseases, including frontotemporal dementia, Alzheimer’s disease, and certain ataxias.
Neurons are especially sensitive to RNA imbalance because they are long-lived cells that rely heavily on precise control of gene expression. Even small disruptions in RNA quality control can have cumulative, devastating effects over time.
This study strengthens the idea that maintaining RNA integrity is not just a background process, but a central pillar of neuronal survival.
Looking Ahead
With this research, scientists have identified a previously underappreciated connection between TDP-43 dysfunction, impaired UPF1 activity, and RNA decay failure in ALS. The discovery provides a clearer molecular framework for understanding how ALS progresses at the cellular level.
As researchers continue to investigate which RNAs become toxic and how they damage motor neurons, the hope is that these insights will lead to more targeted and effective therapies—ones that address the root causes of ALS rather than its downstream consequences.
Research paper:
https://doi.org/10.1016/j.neuron.2025.11.001