How mRNA Vaccines and Lipid Nanoparticles Work Together to Build Powerful Antibody Responses
mRNA vaccines, best known for their role in fighting COVID-19, have reshaped how scientists think about vaccination. They are fast to design, highly adaptable, and remarkably effective. But for years, one key question lingered: how exactly do mRNA vaccines trigger such strong and long-lasting antibody responses inside the body?
A new study published in Cell by researchers from the Perelman School of Medicine at the University of Pennsylvania offers some of the clearest answers yet. The research reveals that mRNA vaccines are not just passive carriers of genetic instructions. Instead, their two main components — the mRNA itself and the lipid nanoparticles (LNPs) that deliver it — actively cooperate to shape the immune response, especially within structures called germinal centers, where high-quality antibodies are made.
Below is a detailed breakdown of what the researchers found, why it matters, and what it could mean for the future of vaccine design.
What Are Germinal Centers and Why Do They Matter?
Germinal centers are specialized structures inside lymph nodes and the spleen. They form temporarily after vaccination or infection and act as training grounds for B cells, the immune cells responsible for making antibodies.
Inside germinal centers, B cells:
- Improve the affinity of their antibodies
- Undergo selection, where only the best antibody producers survive
- Generate long-lived plasma cells and memory B cells, which provide lasting protection
Strong and sustained germinal center responses are closely linked to durable immunity, which is why understanding how vaccines trigger them is so important.
The Study at a Glance
The research team, led by Michela Locci, Ph.D., associate professor of microbiology, investigated how different elements of mRNA vaccines contribute to germinal center formation. Their findings challenge the idea that vaccine components play purely mechanical roles.
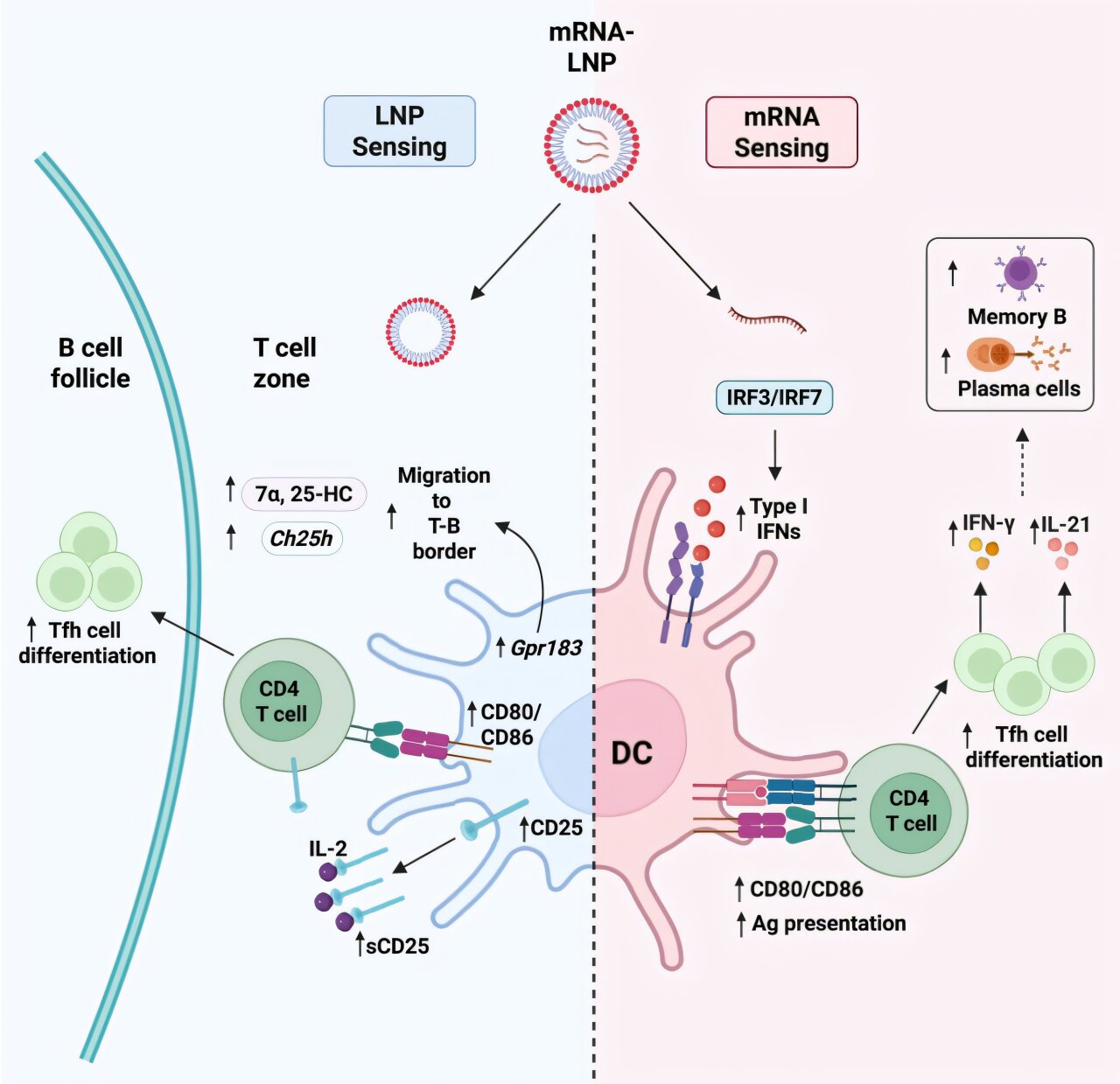
Instead, the study shows that both the mRNA and the lipid nanoparticles act as built-in immune stimulators, each triggering specific signals that guide immune cells toward an effective antibody response.
The Role of mRNA Is More Active Than Previously Thought
mRNA used in vaccines is chemically modified. These modifications were designed to:
- Reduce excessive inflammation
- Prevent the immune system from destroying the mRNA too quickly
- Improve protein production
For a long time, scientists assumed that these modifications made mRNA mostly “invisible” to immune sensors. This study shows that assumption was incomplete.
The researchers found that modified mRNA still triggers low but biologically meaningful levels of type I interferons. These interferons are signaling molecules that help coordinate immune responses.
Importantly:
- The interferon response is controlled, not excessive
- It plays a key role in steering helper T cells toward becoming T follicular helper (Tfh) cells
- Tfh cells are essential for supporting B cells inside germinal centers
In short, the mRNA does more than encode a viral protein. It also provides subtle immune instructions that help shape the quality of the antibody response.
Lipid Nanoparticles Do More Than Deliver mRNA
Lipid nanoparticles are often described as delivery vehicles that protect mRNA and help it enter cells. This study highlights a much more active role.
The researchers discovered that LNPs directly stimulate immune cells, particularly dendritic cells, which are crucial for initiating adaptive immune responses.
Specifically, LNPs:
- Activate dendritic cells
- Influence where these cells position themselves within lymph nodes
- Help dendritic cells deliver the right signals to T cells
This positioning matters. When dendritic cells are located in the correct regions of lymph nodes, they are better able to guide T cells toward becoming helper cells that support B-cell maturation.
The result is a stronger and more organized germinal center response.
IL-1 Emerges as an Independent and Critical Signal
Beyond interferons and dendritic cell activation, the study uncovered another important player: interleukin-1 (IL-1).
IL-1 is a well-known inflammatory cytokine, but its role in mRNA vaccine responses was not fully understood. The researchers found that:
- IL-1 acts independently of type I interferons
- It is essential for shaping effective germinal center responses
- Blocking IL-1 signaling significantly impaired antibody-producing structures
This finding suggests that multiple immune pathways operate in parallel, ensuring that germinal centers form even if one signal is weaker than expected.
How These Signals Work Together
One of the most important conclusions of the study is that no single component works alone. Instead, the immune response emerges from cooperation:
- mRNA induces controlled interferon signaling that guides helper T-cell differentiation
- LNPs activate and position dendritic cells for optimal immune instruction
- IL-1 provides an additional layer of support for germinal center development
Together, these signals promote:
- Robust T follicular helper cell responses
- Strong germinal center formation
- Production of high-affinity antibodies
- Development of long-lived immune memory
This coordinated activity helps explain why mRNA vaccines perform so well across different populations.
Why This Matters for Future mRNA Vaccines
Understanding these mechanisms opens the door to more precise vaccine design. Rather than relying on trial and error, scientists can now think about intentionally tuning immune signals.
Potential applications include:
- Designing LNP formulations that fine-tune dendritic cell activation
- Adjusting mRNA features to optimize interferon signaling
- Balancing immune stimulation to improve efficacy while minimizing side effects
These insights could be especially valuable for:
- Vaccines against rapidly mutating viruses
- Cancer vaccines that require strong immune activation
- Personalized mRNA therapies
Extra Context: Why mRNA Vaccines Are So Adaptable
One reason mRNA vaccines stand out is their platform nature. The same delivery system can be reused while swapping out the genetic instructions. This study reinforces that idea by showing how the platform itself contributes to immune education.
Unlike traditional vaccines that rely on added adjuvants, mRNA vaccines carry self-contained immune cues within their structure. This built-in coordination may explain their speed, scalability, and effectiveness.
A Clearer Picture of Immune Engineering
This research adds an important piece to the puzzle of how modern vaccines work. It shows that mRNA vaccines are not just about producing an antigen. They are carefully balanced systems where delivery materials and genetic instructions jointly shape immunity.
By revealing how germinal centers are instructed at a molecular level, the study offers a roadmap for improving vaccines that aim to produce strong, lasting antibody protection.
Research paper reference:
https://doi.org/10.1016/j.cell.2025.11.023