How Tumor Metabolism Influences Cancer Drug Effectiveness and Could Shape Precision Chemotherapy
Chemotherapy has always walked a fine line. The goal is simple in theory but incredibly complex in practice: destroy cancer cells without harming healthy ones. For decades, scientists have been searching for ways to make cancer drugs more selective, more precise, and less toxic. Now, a new study published in Nature Communications (2025) offers an important step forward by revealing a direct link between tumor metabolism and how well certain cancer drugs actually work inside cells.
This research brings together metabolism, genetics, drug design, and cutting-edge live-cell technology to explain why some drugs are highly effective in specific tumors—and far less effective in others. The findings may significantly influence how precision chemotherapy is developed in the future.
The Central Player: PRMT5 and Why It Matters in Cancer
At the heart of this research is a protein called PRMT5 (Protein Arginine Methyltransferase 5). PRMT5 plays a key role in regulating gene expression by modifying other proteins through a process known as arginine methylation. This function is essential for normal cellular activity, but it also makes PRMT5 critically important for cancer cell survival.
Because many cancer cells rely heavily on PRMT5 to maintain uncontrolled growth, it has long been considered a top target for cancer drug discovery. However, targeting PRMT5 has been challenging. Since it is also active in healthy cells, drugs that block it often cause significant side effects, limiting how much patients can safely receive.
This is where tumor metabolism enters the picture.
MTAP Deletion and a Metabolic Weakness in Cancer Cells
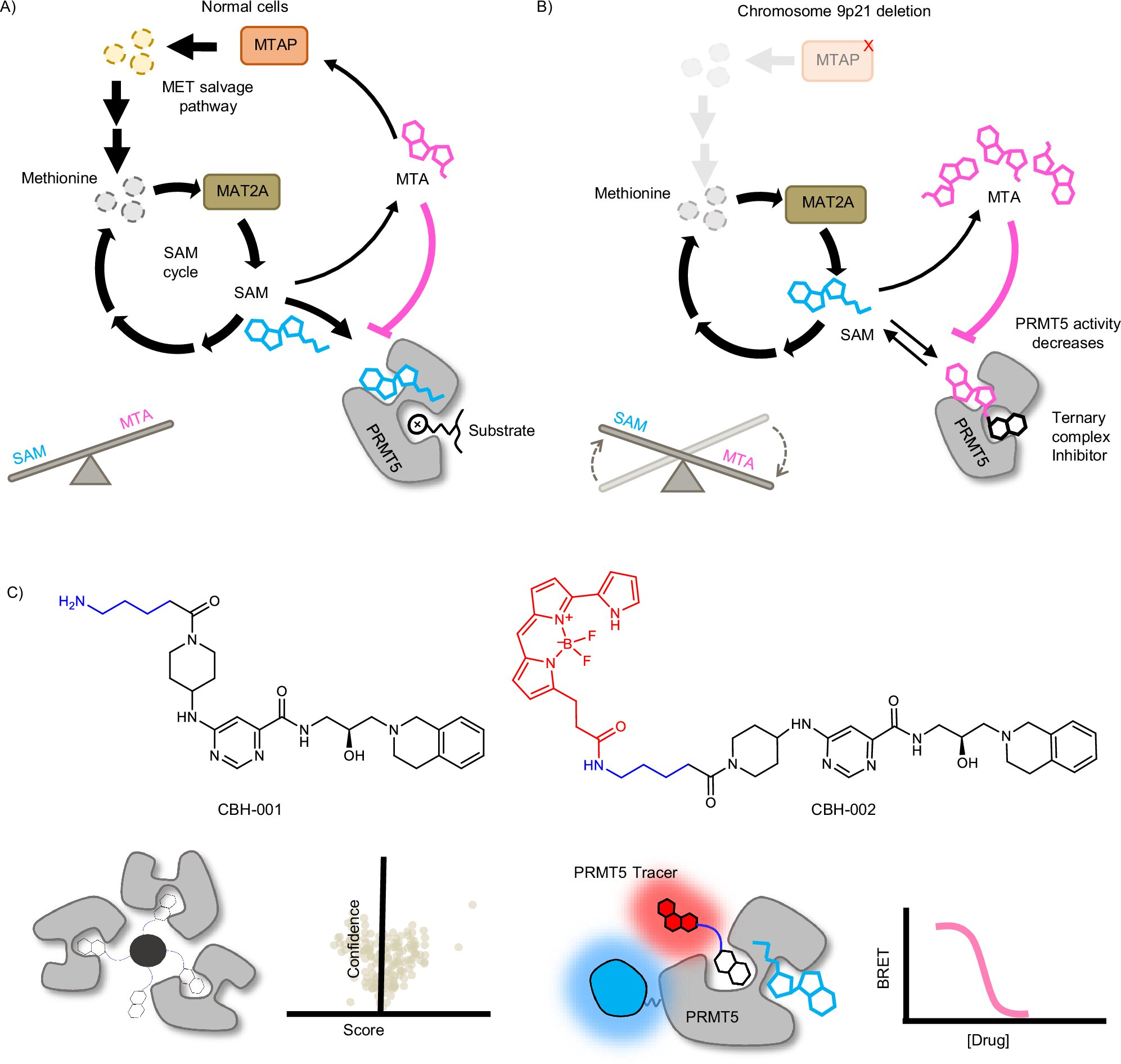
Roughly 10% to 15% of all cancers carry a deletion in a gene known as MTAP (methylthioadenosine phosphorylase). When MTAP is missing, cancer cells can no longer properly break down a molecule called MTA (methylthioadenosine). As a result, MTA accumulates inside these tumor cells.
In healthy cells, PRMT5 normally interacts with a molecule called SAM (S-adenosylmethionine), which serves as a methyl donor. But in MTAP-deleted cancer cells, the buildup of MTA changes the biochemical environment. PRMT5 begins interacting differently, forming a distinct enzyme–metabolite complex that does not exist in normal tissue.
This metabolic difference creates a unique vulnerability. If a drug could selectively target PRMT5 only when it is bound to MTA, it could potentially attack cancer cells while leaving healthy cells largely unharmed.
Measuring Drug Engagement Inside Living Cells
One of the major challenges in cancer research has been figuring out how drugs behave inside live cells, not just in test tubes. Many compounds look promising in biochemical assays but fail in real biological environments because they do not engage their targets effectively under actual cellular conditions.
To address this, the research team used a bioluminescent technology known as NanoBRET (Bioluminescence Resonance Energy Transfer). This system allows scientists to measure drug–target engagement in real time within living cells.
Using NanoBRET, the researchers created a genetically encoded PRMT5–NanoLuc biosensor that emits light when a drug binds to PRMT5. This made it possible to directly compare how different inhibitors interact with PRMT5 under varying metabolic conditions.
The Role of CBH-001 and CBH-002 Chemical Probes
A key breakthrough in the study was the development of two chemical probes, CBH-001 and CBH-002. These probes were designed to bind to PRMT5 and report on drug engagement using the NanoBRET system.
The University of Oxford team developed CBH-002, a cell-permeable BRET probe that could function inside live cells. What made CBH-002 especially powerful was its sensitivity to metabolic context. The probe could detect whether PRMT5 was interacting with SAM or MTA, effectively turning it into a metabolic biosensor.
This allowed the researchers to show, for the first time, that certain PRMT5 inhibitors bind much more effectively when PRMT5 is associated with MTA rather than SAM.
Uncompetitive Inhibition Explained Clearly
One of the most important findings of the study is the identification of an uncompetitive (or cooperative) inhibition mechanism occurring inside live cancer cells.
In simple terms, uncompetitive inhibitors only bind to an enzyme after the enzyme has already bound to another molecule. In this case, some PRMT5 inhibitors only bind efficiently when PRMT5 is already bound to MTA, which accumulates specifically in MTAP-deleted cancer cells.
This is a powerful concept because it means drug activity is naturally restricted to tumor tissue. Normal cells, which do not accumulate MTA, are far less affected. The study marks the first direct characterization of this type of inhibitor mechanism in living cells, rather than inferred from indirect biochemical data.
Why This Matters for Precision Chemotherapy
This research provides a clear mechanistic explanation for why some PRMT5 inhibitors show exceptional potency in MTAP-deleted cancers. More importantly, it shows how tumor metabolism directly influences drug efficacy, not just drug uptake or resistance.
From a therapeutic standpoint, this opens the door to designing highly selective cancer drugs that exploit metabolic differences rather than just genetic mutations. Such drugs could:
- Reduce damage to healthy tissue
- Allow higher effective doses
- Minimize dose-limiting toxicities
- Improve patient outcomes
The ability to directly observe drug engagement in live cells also gives researchers a powerful tool to screen and optimize future cancer therapies more accurately.
Broader Context: Cancer Metabolism as a Drug Target
Cancer metabolism has become an increasingly important field over the past decade. Tumors often rewire metabolic pathways to support rapid growth, survive stress, and evade treatment. These metabolic changes can create specific dependencies that healthy cells do not share.
The MTAP–PRMT5 relationship is a strong example of this concept in action. Instead of targeting cancer broadly, researchers are now learning how to exploit context-specific vulnerabilities that arise from metabolic rewiring.
As drug discovery moves forward, metabolism-aware approaches like this one could become a cornerstone of next-generation oncology treatments.
Collaboration and Technology Driving the Discovery
This work was the result of a broad collaboration involving Stony Brook University’s Center for Advanced Discovery of Drug Action, the University of Oxford’s Center for Medicines Discovery, Boston University, and the Promega Corporation. Promega’s NanoBRET Target Engagement technology played a crucial role in enabling live-cell measurements that were previously impossible.
The study demonstrates how combining chemical biology, metabolic science, and advanced biosensing technologies can answer long-standing questions in cancer research.
Final Thoughts
This research doesn’t just add another piece to the cancer puzzle—it connects multiple pieces into a clearer picture. By showing how tumor-specific metabolism controls drug effectiveness, the study provides both a scientific explanation and a practical roadmap for developing more precise cancer therapies.
As precision medicine continues to evolve, insights like these will likely shape how future chemotherapy drugs are designed, tested, and prescribed.
Research Paper:
https://www.nature.com/articles/s41467-025-65558-6