Light-Activated Engineered Material Breaks Down PFAS and Other Stubborn Water Pollutants
Clean drinking water is becoming harder to secure as industrial chemicals, pharmaceuticals, and synthetic dyes continue to contaminate natural water sources. Among the most troubling of these pollutants are per- and polyfluoroalkyl substances (PFAS), often called “forever chemicals” because they resist natural breakdown and accumulate in the environment and the human body. In a promising new development, materials scientists at Rice University, working with collaborators, have engineered a light-activated material capable of destroying PFAS and several other difficult contaminants directly in water.
This breakthrough, published in the journal Materials Today, introduces a new kind of metal-free photocatalytic surface that relies only on light to trigger chemical reactions that dismantle pollutants at the molecular level. The approach is not only effective but also environmentally safer and potentially more affordable than many existing water-treatment technologies.
What Makes PFAS So Difficult to Remove
PFAS are widely used in products like nonstick cookware, waterproof fabrics, firefighting foams, food packaging, and electronics manufacturing. Their usefulness comes from the extremely strong carbon-fluorine bonds within their molecular structure. Unfortunately, these same bonds make PFAS incredibly persistent once they enter water, soil, or living organisms.
Traditional water-treatment methods often capture PFAS rather than destroy them, transferring the chemicals from water to filters or absorbent materials. This creates a secondary disposal problem, since the PFAS still exist and must be handled carefully to avoid re-contamination. A technology that can actually break PFAS apart instead of merely collecting them has long been a goal in environmental science.
The Core Technology Behind the New Material
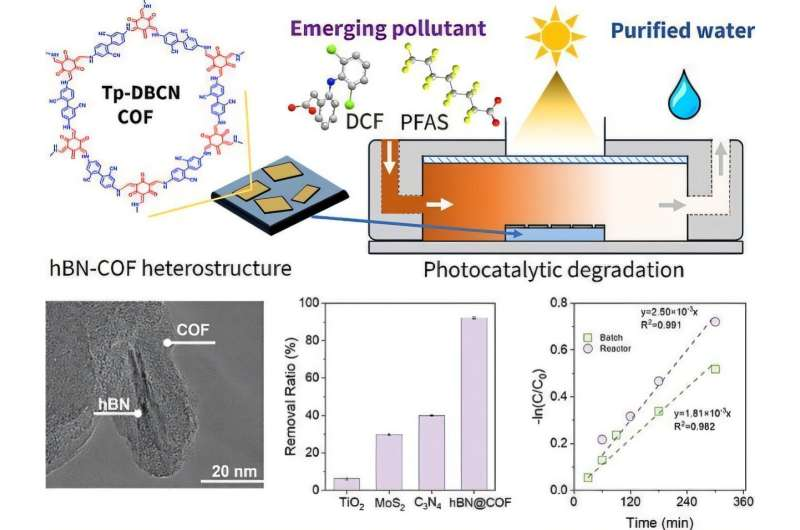
At the heart of this research is a class of materials called covalent organic frameworks, or COFs. COFs are crystalline, porous materials built from lightweight elements such as carbon, nitrogen, and boron. Their defining features include high surface area, tunable chemical structure, and strong ability to interact with light.
When exposed to light, COFs can act as photocatalysts. Light energy excites electrons in the material, separating negatively charged electrons from positively charged “holes.” This separation of charge creates reactive sites capable of driving chemical reactions, including the breakdown of stubborn pollutants.
However, COFs alone are not always efficient enough to rapidly destroy contaminants like PFAS. To solve this, the Rice University team combined COFs with another advanced material: hexagonal boron nitride (hBN).
Why Hexagonal Boron Nitride Matters
Hexagonal boron nitride is a two-dimensional material structurally similar to graphene but composed of boron and nitrogen atoms. It is lightweight, chemically stable, and considered safe for environmental applications. Importantly, hBN has electronic properties that make it useful for guiding the movement of electrons.
The challenge was that COFs and hBN do not naturally bond well. Simply mixing them would not create the tight electronic coupling needed for efficient photocatalysis. To overcome this, the researchers used a technique known as defect engineering.
Defect Engineering Creates a Powerful Interface
Defect engineering involves intentionally introducing tiny imperfections into a material to give it new properties. In this case, the researchers etched microscopic defects or “scratches” into the surface of the hBN film. These imperfections acted as anchoring sites, allowing the COF material to grow directly on top of the hBN layer.
This direct growth created a COF/hBN heterostructure, meaning the two materials are tightly connected at the atomic level. The resulting interface allows light-excited electrons and holes to move efficiently in different directions rather than recombining and losing energy. This improved charge separation is what gives the material its strong pollutant-destroying ability.
How the Material Cleans Water
When light shines on the COF/hBN surface, the material generates highly reactive charge carriers. These charges interact with pollutants in the surrounding water, breaking chemical bonds and transforming complex contaminants into simpler, less harmful substances.
In laboratory tests, the material successfully degraded a wide range of pollutants, including:
- PFAS compounds, known for their chemical stability
- Pharmaceutical residues, which increasingly appear in wastewater
- Industrial dyes, often released from textile and manufacturing processes
A key advantage of this system is that it does not rely on toxic metals such as titanium, copper, or iron, which are common in many photocatalysts and can pose environmental risks of their own.
Tested Under Realistic Water-Flow Conditions
To assess real-world potential, the researchers tested the material in both vertical and horizontal flowing-water reactors, similar to those used in actual water-treatment facilities. Rather than degrading quickly or losing effectiveness, the material remained structurally stable and continued to perform well across multiple cleaning cycles.
This durability is especially important for practical deployment, as water-treatment materials must operate continuously without frequent replacement. The researchers found that the COF/hBN surface maintained its performance while resisting physical and chemical degradation.
Why This Discovery Is a Big Deal
This research demonstrates that a single, metal-free material can destroy multiple classes of hard-to-remove water pollutants using only light. That combination of efficiency, safety, and versatility moves photocatalytic water treatment closer to large-scale implementation.
Because the material is lightweight and does not require rare or hazardous components, it could potentially be integrated into low-cost purification systems, especially in regions where access to advanced water treatment is limited.
A Broader Look at Photocatalytic Water Treatment
Photocatalysis is an expanding field with applications far beyond PFAS removal. Similar light-activated materials are being studied for breaking down pesticides, killing harmful microbes, and even producing clean hydrogen fuel from water. The COF/hBN system adds to growing evidence that engineered nanostructures can address environmental challenges once thought nearly impossible to solve.
However, challenges remain. Delivering light efficiently in murky or highly contaminated water is still a technical hurdle, and long-term performance in complex, real-world water systems will require further testing. Even so, this study marks a significant step forward.
Looking Ahead
As regulations on PFAS tighten worldwide, technologies that destroy rather than store these chemicals will become increasingly valuable. The Rice University team’s work shows that careful material design, combined with smart engineering at the nanoscale, can open new paths toward cleaner, safer water.
With continued development and scaling, light-activated materials like this one could become a core part of next-generation water-treatment systems, helping to finally tackle pollutants that have lingered in our environment for far too long.
Research paper:
https://doi.org/10.1016/j.mattod.2025.11.004