Scientists Discover a Shape-Shifting Cell Channel That Could Lead to More Precise Drugs
Scientists have uncovered a fascinating new detail about how cells communicate with each other, and it could change the way future drugs are designed. At the center of this discovery is a cellular channel called pannexin-1 (PANX1), a gate-like structure embedded in the membranes of cells throughout the human body. This channel plays a crucial role in controlling what substances move in and out of cells, and new research shows that it is far more flexible and sophisticated than scientists once believed.
The study, published in Nature Communications in 2025, reveals that PANX1 is not a rigid tube but a dynamic, shape-shifting molecular valve. It can widen or narrow its opening to allow the passage of very different types of molecules, ranging from tiny ions to much larger signaling compounds. This discovery also points to a new way drugs could be designed to fine-tune cellular communication instead of completely shutting it down.
What PANX1 Does and Why It Matters
PANX1 channels are found in the membranes of many cell types across the body, including nerve cells, immune cells, and muscle cells. Their main job is to help release molecules from inside the cell into the surrounding environment. One of the most important of these molecules is adenosine triphosphate (ATP).
While ATP is widely known as the cell’s internal energy currency, it also acts as a powerful signaling molecule outside the cell. When released, ATP helps cells coordinate essential biological processes such as immune responses, inflammation, wound healing, nerve signaling, and fertility. Because PANX1 controls ATP release, it has been linked to a wide range of health conditions, including cardiovascular disease, neurological disorders, chronic pain, muscular dystrophy, and reproductive issues.
Until now, scientists did not fully understand how PANX1 could allow both small ions and much larger molecules like ATP to pass through the same channel.
The Shape-Shifting Mechanism Inside PANX1
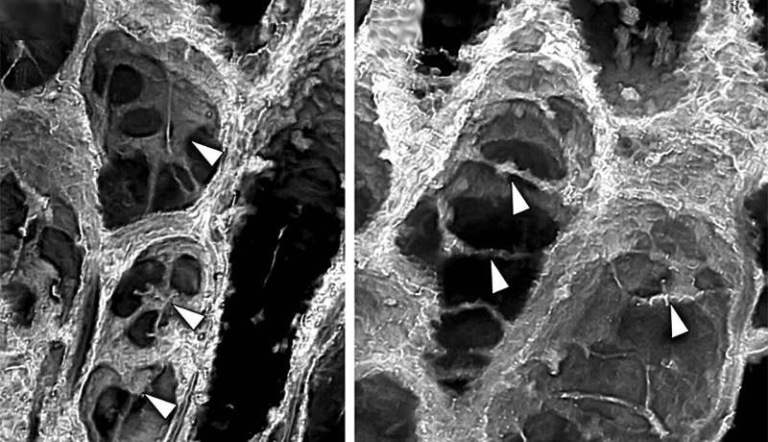
The new study shows that PANX1 can physically change its structure, much like the iris of an eye adjusting to light. Using cryo-electron microscopy (cryo-EM), researchers captured high-resolution images of PANX1 in multiple states. These snapshots revealed that a ring of specific amino acids sits at the outer entrance of the channel and acts as a flexible gate.
When this gate is in a constricted state, only small ions such as chloride can pass through. When the gate dilates, larger molecules like ATP can move out of the cell. This switching between narrow and wide openings allows PANX1 to handle very different cargo sizes without needing separate channels.
At the molecular level, this flexibility is controlled by interactions between key amino acids, including tryptophan and arginine residues, which shift positions to tighten or relax the gate. This elegant mechanism explains how PANX1 can rapidly alternate between different functional modes depending on the cell’s needs.
Hidden Side Tunnels and a New Drug Binding Site
This discovery builds on earlier work from the same research team. In 2020, the scientists created a near-atomic structural map of PANX1 and found that, in addition to the main channel, it contains seven narrow side tunnels branching off from the core pore. This overturned the long-standing belief that all signaling through PANX1 occurred via a single pathway.
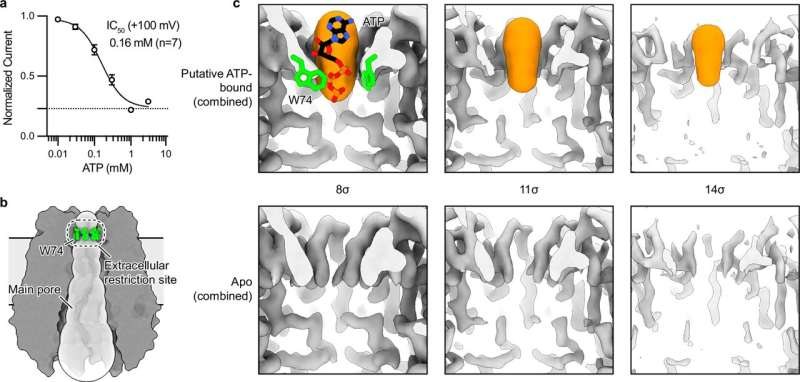
The new study reveals that one of these side tunnels contains a previously unknown drug-binding pocket. This finding turned out to be especially important when researchers examined how certain drugs interact with PANX1.
An Old Malaria Drug with a New Role
One of the most surprising findings of the study involves mefloquine, an FDA-approved antimalarial drug that has been used for decades. The researchers discovered that mefloquine binds to the newly identified pocket near a side tunnel of PANX1 rather than blocking the main channel.
Instead of inhibiting PANX1, mefloquine enhances its activity, allowing more ions to flow through the channel. This makes it the first known compound shown to positively modulate PANX1 function rather than shutting it down completely.
This distinction is critical. Most existing PANX1 inhibitors work by blocking the main pore, which stops both harmful and normal signaling. That broad shutdown often leads to unwanted side effects. By contrast, targeting a side pocket allows for fine control, opening the door to drugs that adjust PANX1 activity up or down depending on therapeutic needs.
Why This Matters for Drug Development
Because PANX1 is involved in so many biological processes, it has long been considered a promising drug target. However, progress has been limited by the lack of selective compounds. The identification of a new, non-blocking drug-binding site provides a blueprint for precision medicines that could regulate cellular communication without disrupting healthy function.
This approach could be particularly valuable for treating conditions tied to abnormal ATP signaling, including inflammatory diseases, neurological disorders, and chronic pain. Rather than silencing PANX1 entirely, future drugs could rebalance its activity, restoring normal signaling patterns.
How the Research Was Done
The study combined multiple advanced techniques to build a complete picture of PANX1 behavior. Cryo-EM was used to visualize structural changes in the channel at near-atomic resolution. Electrophysiological recordings measured how ions move through PANX1 under different conditions. Computer simulations helped model how molecules travel through the main channel and side tunnels.
Together, these methods allowed researchers to connect structural changes with functional outcomes, revealing how PANX1’s shape-shifting ability directly affects what it allows to pass.
PANX1 in the Bigger Biological Picture
PANX1 belongs to a family of large-pore channels that are distinct from traditional ion channels. Unlike highly selective channels that allow only one type of ion to pass, PANX1 is designed for versatility, enabling rapid communication between cells and tissues.
Its ability to release ATP makes it a central player in purinergic signaling, a communication system used throughout the body. Disruptions in this system have been linked to inflammation, neurodegeneration, and immune dysfunction, which explains why PANX1 continues to attract attention from researchers across multiple fields.
What Comes Next
The discovery that PANX1 can be positively modulated rather than simply blocked represents a major shift in how scientists think about targeting this channel. With a detailed structural map and a newly identified drug-binding pocket, researchers now have the tools needed to design highly selective therapies that adjust cellular signaling with greater precision.
As more compounds are developed to interact with PANX1’s side tunnels, this research could lead to safer and more effective treatments for a wide range of diseases rooted in disrupted cell-to-cell communication.
Research Paper:
Structural basis of PANX1 permeation and positive modulation by mefloquine – Nature Communications (2025)
https://www.nature.com/articles/s41467-025-66028-9