Human Cytomegalovirus Can Rewire the Cell’s Internal Structure to Promote Infection
Human cytomegalovirus, often shortened to HCMV, is one of those viruses that most people never think about, even though it infects a large portion of the global population. A recent study has now revealed something striking about how this virus behaves inside the body. Researchers have discovered that HCMV can actively reprogram the internal architecture of infected cells, allowing it to control nuclear movement, reshape the cell, and promote infection in ways that were not fully understood before.
This research comes from the laboratory of Derek Walsh, a professor of Microbiology-Immunology, and was published in the prestigious journal Proceedings of the National Academy of Sciences (PNAS). The findings provide new insight into how viruses can manipulate the very core of a cell to support their own survival and spread.
Understanding human cytomegalovirus and why it matters
HCMV is a DNA virus belonging to the herpesvirus family. Like other herpesviruses, once it infects a person, it typically remains in the body for life. In healthy individuals, the immune system usually keeps the virus under control, meaning most people never experience noticeable symptoms.
However, the situation is very different for certain groups. HCMV can cause severe illness in people with weakened immune systems, such as organ transplant recipients, cancer patients undergoing chemotherapy, and individuals living with advanced HIV. It is also the leading infectious cause of congenital birth defects, according to public health authorities. Babies infected before birth can develop hearing loss, vision problems, and neurological impairments.
Because of these risks, scientists have long been interested in understanding exactly how HCMV operates at the cellular level.
Why scientists focused on nuclear movement
One of the most recognizable features of HCMV infection is the way infected cells look under a microscope. The cell nucleus becomes enlarged, distorted, and misshapen, especially during later stages of infection. This unusual nuclear appearance has been known for decades, but the precise mechanisms behind it were unclear.
The research team wanted to understand how HCMV interacts with the cytoskeleton, the internal framework of the cell made up of structures such as microtubules and actin filaments. These components are responsible for maintaining cell shape, positioning the nucleus, and enabling cell movement. Since nuclear positioning is essential for many cellular processes, any viral control over this system could be extremely powerful.
How the researchers studied infected cells
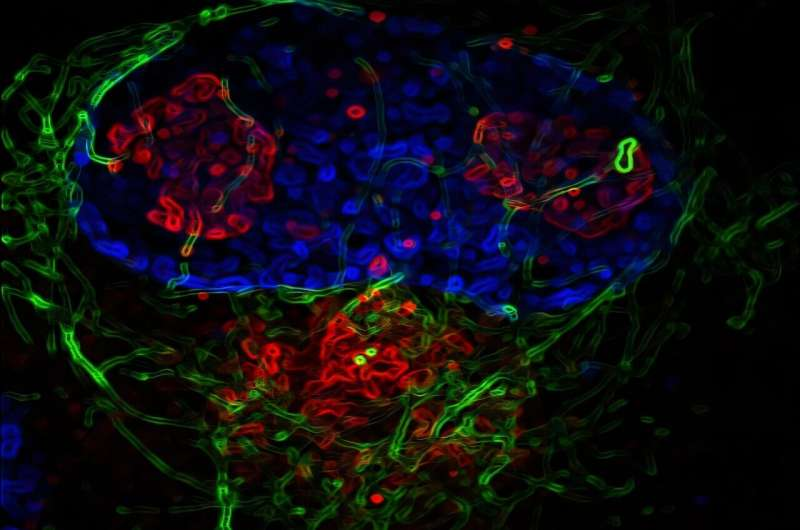
To observe these changes in real time, the scientists used extended live-cell imaging, also known as time-lapse microscopy. This approach allowed them to track infected cells continuously over several days, which is especially important because HCMV replicates slowly compared to many other viruses.
The virus and host cells were engineered to express fluorescent markers, making it possible to see how the nucleus, cytoskeleton, and viral structures changed during infection. The researchers combined this with fixed-cell imaging, which provides highly detailed snapshots of cellular structures at specific points in time.
This combination of techniques gave them a clear view of how HCMV gradually reshapes the cell from the inside.
A viral kinase disrupts Lamin A/C
One of the most important discoveries was that HCMV encodes a viral kinase that directly disrupts the organization of Lamin A/C. Lamin A/C is a network of filament proteins lining the inner surface of the nuclear membrane. Its job is to provide structural support, organize chromatin, and help the nucleus maintain its shape.
Lamin A/C also plays a key role in connecting the nucleus to the cytoskeleton. It interacts with proteins that physically link the nucleus to structures like microtubules, allowing the nucleus to move when a cell migrates.
By disrupting Lamin A/C, HCMV weakens this structural framework. As a result, the nucleus becomes more flexible, distorted, and easier to reposition within the cell.
How SUN proteins and the LINC complex are affected
The study also revealed that HCMV interferes with a group of proteins called SUN1 and SUN2, particularly SUN2. These proteins are part of the LINC complex, which stands for “linker of nucleoskeleton and cytoskeleton.”
The LINC complex acts as a molecular bridge, connecting Lamin A/C inside the nucleus to cytoskeletal filaments outside the nucleus. This system is essential for maintaining proper nuclear positioning and for coordinating nuclear movement during cell migration.
The researchers found that HCMV actively downregulates SUN2, reducing its levels in infected cells. This disruption prevents SUN2 and related proteins from interfering with the formation of specialized microtubules that the virus depends on.
The role of microtubules in viral control
Microtubules are long, tube-like structures that act as tracks for transporting materials inside the cell. They are also crucial for positioning the nucleus, especially when cells move.
In HCMV-infected cells, the virus promotes the formation of acetylated microtubules, a more stable form of microtubules that support sustained nuclear movement. By weakening Lamin A/C and reducing SUN2, the virus removes obstacles that would normally limit microtubule-driven nuclear motion.
This allows HCMV to take control of how the nucleus moves and where it is positioned within the cell, particularly during later stages of infection when infected cells begin to migrate.
Why nuclear movement and cell migration matter
Cell migration is a normal biological process involved in wound healing, immune responses, and tissue development. However, during HCMV infection, this movement appears to benefit the virus.
By controlling nuclear positioning and cell migration, HCMV may enhance its ability to spread within tissues, interact with neighboring cells, or establish environments that favor viral replication. The distorted nucleus seen in infected cells is not just a visual oddity but a functional outcome of viral manipulation.
Broader implications for virology and medicine
This research highlights just how deeply viruses can interfere with host cell biology. Rather than simply hijacking basic replication machinery, HCMV rewires entire cellular networks, including nuclear architecture and cytoskeletal dynamics.
These findings may have implications beyond HCMV alone. Other viruses could use similar strategies to manipulate nuclear movement and cell structure. Understanding these mechanisms opens the door to identifying new therapeutic targets, especially viral kinases or host proteins that viruses depend on.
While clinical applications may still be years away, studies like this demonstrate the value of fundamental research in revealing vulnerabilities that could eventually be exploited by antiviral drugs.
Expanding our understanding of nuclear structure and disease
Beyond virology, this work also contributes to broader cell biology. Lamin A/C and LINC complexes are involved in a range of human diseases, including muscular dystrophies, cardiomyopathies, and premature aging disorders. Seeing how a virus manipulates these systems offers new insight into their normal functions and weaknesses.
By studying how HCMV dismantles and repurposes these structures, scientists gain a clearer picture of how nuclear architecture is maintained and how it can fail under stress.
Research paper reference:
https://www.pnas.org/doi/10.1073/pnas.2507831122