Scientists Discover a Short-Lived Src Kinase State That Is Crucial for Cell Migration and T-Cell Function
Scientists at St. Jude Children’s Research Hospital have uncovered a previously hidden and extremely short-lived state of Src family kinases that turns out to be absolutely essential for normal cell behavior. This discovery sheds new light on how kinases work at a molecular level and helps explain critical biological processes such as cell migration and T-cell activation. The findings were published in the journal Science in December 2025 and are already reshaping how researchers think about kinase regulation and therapeutic targeting.
Understanding Kinases and Why Src Matters
Kinases are enzymes that act as master regulators inside cells. Their main job is to transfer a phosphate group from ATP (adenosine triphosphate) to other proteins, a process known as phosphorylation. This single chemical modification can dramatically change how a protein behaves — turning signals on or off, altering protein interactions, or directing cells to move, divide, or respond to external stimuli.
Among the many kinase families in human cells, Src family kinases (SFKs) play especially important roles. Members of this family — including Src, Lck, and Hck — are deeply involved in processes like immune signaling, cytoskeletal remodeling, and cellular movement. Because of their central role, SFKs have long been studied in cancer, immune disorders, and inflammatory diseases.
For decades, researchers believed that kinases like Src primarily switched between two main shapes: active and inactive. However, the new research shows that this view is incomplete.
A Hidden Intermediate State Comes Into Focus
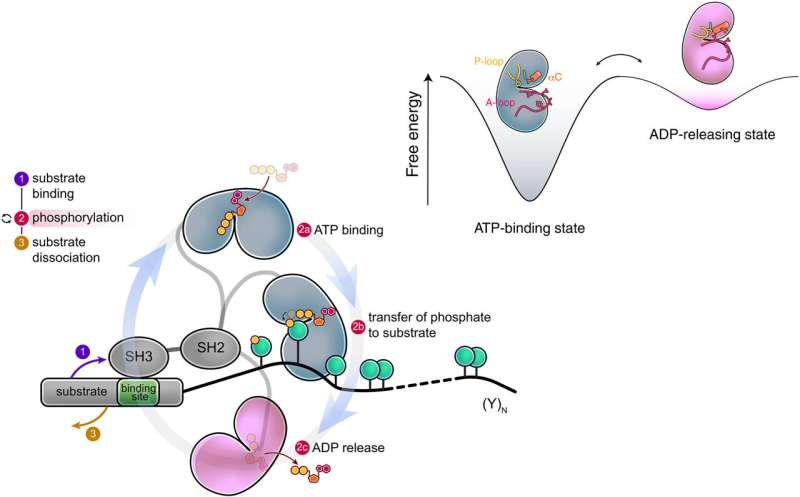
The St. Jude research team, led by Charalampos Babis Kalodimos, discovered that Src kinases pass through a previously invisible intermediate state during their catalytic cycle. This state exists only briefly, which explains why it had gone unnoticed for so long, but its impact is profound.
This intermediate state acts as a molecular fast-track that allows the kinase to rapidly release ADP (adenosine diphosphate) after phosphorylation. ADP is the waste product left behind when ATP donates its phosphate group. If ADP lingers too long inside the kinase, the enzyme stalls and becomes inefficient.
By briefly adopting this hidden shape, Src can eject ADP quickly and bind a new ATP molecule almost immediately. This rapid turnover allows the kinase to remain attached to its target protein and continue working without interruption.
Why Processive Phosphorylation Is So Important
Many cellular processes depend on processive phosphorylation, where a kinase adds multiple phosphate groups to a single target protein in one continuous interaction. This is especially important in pathways that control cell migration and immune activation, where precise and efficient signaling is required.
Without fast ADP release, the kinase would detach too soon, leaving the target protein only partially modified. The newly discovered intermediate state solves this problem by enabling continuous, efficient phosphorylation while the kinase remains docked to its substrate.
This mechanism helps explain how Src family kinases achieve the speed and precision needed for complex biological tasks.
How the Hidden State Was Discovered
Capturing such a fleeting molecular state required advanced techniques. The researchers used nuclear magnetic resonance (NMR) spectroscopy, a powerful method capable of detecting subtle and short-lived changes in protein structure.
This work was conducted as part of the St. Jude Blue-Sky Kinases initiative, a research effort focused on uncovering overlooked or invisible aspects of kinase biology. By closely examining the dynamic movements of Src kinases, the team was able to map out this hidden intermediate state and understand its functional role.
Evidence From Cell Migration and T-Cell Studies
To confirm that this intermediate state truly matters in living cells, the researchers introduced targeted mutations that eliminated the hidden state without completely shutting down kinase activity.
The results were striking:
- Cell migration, which depends heavily on Src and Hck, was significantly impaired.
- T-cell activation and regulation, controlled largely by Lck, was also disrupted.
These findings showed that the hidden state is not just a biochemical curiosity but a biological necessity. Without it, cells lose their ability to move properly and immune cells fail to function as they should.
Why This Discovery Matters for Medicine
Kinases are among the most heavily targeted proteins in modern drug development. Many cancer therapies and immune-modulating drugs work by inhibiting kinase activity. However, most existing drugs focus on stabilizing either the active or inactive state of a kinase.
This research introduces a new idea: therapeutically targeting transient conformational states.
By designing drugs that specifically affect the newly discovered intermediate state, it may be possible to fine-tune kinase activity rather than simply turning it on or off. This approach could lead to treatments with greater precision and fewer side effects.
Implications for Immunotherapy and CAR T Cells
The discovery also has implications for adoptive immunotherapies, including CAR T-cell therapy, which relies on engineered T cells to attack cancer. Src family kinases are crucial for T-cell signaling, and subtle changes in kinase behavior can dramatically affect therapeutic outcomes.
Understanding and potentially manipulating this hidden state could help researchers optimize CAR T-cell performance, improving their effectiveness while reducing harmful immune reactions.
Beyond Src: A Broader Kinase Landscape
One of the most intriguing aspects of this study is its broader message. Src family kinases are not unique in their complexity. The researchers believe that many other kinases likely possess hidden or invisible states that remain undiscovered.
Traditional structural biology often focuses on stable protein conformations, but this work highlights the importance of dynamic, short-lived states that are just as essential for function.
This opens the door to a new era of kinase research, where understanding motion and flexibility becomes just as important as mapping static structures.
A Shift in How We Understand Cellular Signaling
At its core, this discovery reshapes our understanding of how signals are transmitted inside cells. Rather than acting as simple on-off switches, kinases operate through complex, finely tuned conformational cycles that ensure speed, accuracy, and adaptability.
The identification of this hidden Src kinase state is a reminder that even well-studied proteins can still surprise us — and that some of the most important biological mechanisms operate in moments so brief they are almost invisible.
Research Paper Reference:
https://www.science.org/doi/10.1126/science.adw8310