Hidden Molecular Switch in the Body Controls Taste, Metabolism, and Gut Function
Scientists at Northwestern University have uncovered a previously unknown molecular mechanism inside a protein that plays a crucial role in how the body senses taste, regulates blood sugar, and maintains gut health. This discovery centers on a protein called TRPM5, revealing that it contains a hidden control site that can both activate and shut down the protein depending on which molecule interacts with it.
What makes this finding especially important is that it challenges long-standing assumptions about how TRPM5 works and opens new possibilities for drug development aimed at conditions like type 2 diabetes, obesity, and gut inflammation.
What Is TRPM5 and Why It Matters
TRPM5, short for Transient Receptor Potential Melastatin 5, is an ion channel protein found in many types of cells throughout the body. Ion channels act as microscopic gates that allow charged particles to pass in and out of cells, helping cells communicate and respond to their environment.
TRPM5 is best known for its role as a signal amplifier. When it opens, sodium ions flow into the cell, strengthening electrical signals that control important biological processes. These include:
- Taste perception in the tongue, particularly sweet, bitter, and umami flavors
- Insulin release from pancreatic beta cells after eating
- Nutrient sensing and immune defense in the gut
Because TRPM5 connects sensory signals to metabolic and immune responses, it sits at the crossroads of taste, metabolism, and gut function.
The Old Model Versus the New Discovery
For years, scientists believed that TRPM5 could only be activated by one trigger: an increase in calcium levels inside the cell. Calcium binding was thought to be the sole on-switch that opened the channel and allowed ions to pass through.
The new research overturns that idea.
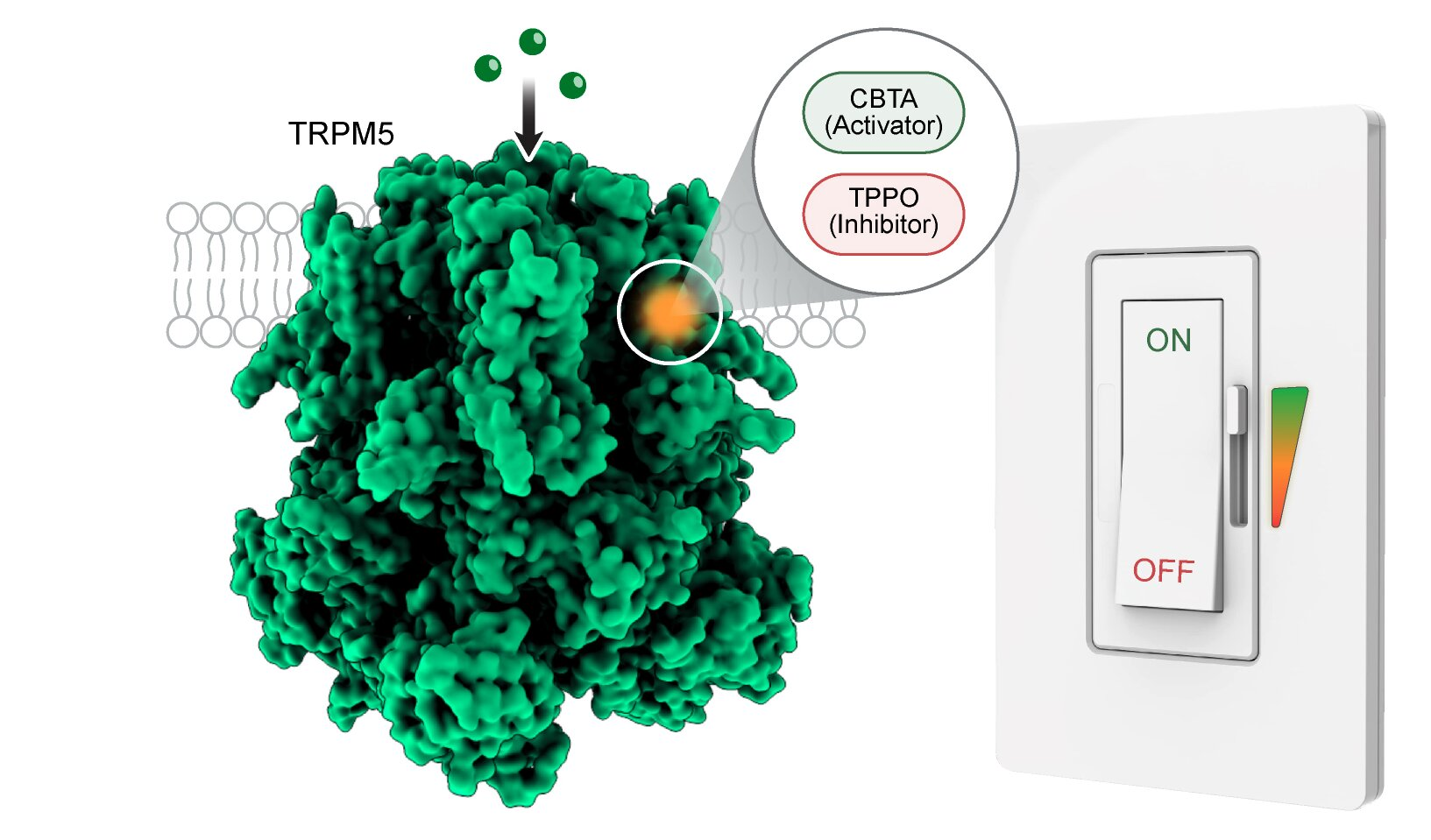
Using advanced structural and functional techniques, researchers discovered that TRPM5 contains a hidden molecular pocket that can be directly controlled by small molecules, without relying on calcium at all. This pocket acts like a dual-purpose control switch, capable of either turning the channel on or locking it shut.
A Dual-Use Control Pocket Inside TRPM5
The newly discovered control site is located within the S1–S4 domain of the TRPM5 protein, a region previously not known to serve this function. What makes this pocket remarkable is its versatility.
The researchers identified two small molecules that bind to the exact same pocket but produce opposite effects:
- CBTA, which activates TRPM5 and opens the channel
- TPPO, which inhibits TRPM5 and keeps the channel closed
Even though these molecules are structurally similar and occupy the same binding site, they induce different conformations in the protein. One acts like an accelerator, while the other works like a brake.
This discovery shows that a single allosteric site can integrate activation, modulation, and inhibition in one place, something that had not been observed before in TRPM5.
How the Switch Enhances Calcium Sensitivity
Another important finding is that when TRPM5 is activated by a molecule like CBTA, it becomes extra sensitive to calcium. In this state, even very small calcium fluctuations can strongly activate the channel.
This means the hidden pocket does more than simply turn TRPM5 on or off. It fine-tunes how responsive the protein is, effectively supercharging its activity under the right conditions. This layered control mechanism adds a new level of complexity to how cells regulate electrical signaling.
Implications for Diabetes and Metabolic Health
TRPM5 plays a key role in pancreatic beta cells, where it helps amplify electrical signals that trigger insulin release after meals. Reduced TRPM5 activity has been linked to impaired insulin secretion and poor glucose control.
By identifying molecules that can directly activate TRPM5, scientists now have a potential pathway to boost insulin production in people with metabolic disorders. Drugs designed to target this newly discovered pocket could help improve blood sugar regulation without interfering with other calcium-dependent processes.
This makes TRPM5 an attractive target for future therapies aimed at type 2 diabetes and obesity.
Effects on Taste and Appetite
Beyond metabolism, TRPM5 is central to taste signaling. It helps transmit information about sweet, bitter, and umami flavors from taste receptor cells to the nervous system.
Modulating TRPM5 activity could allow scientists to alter taste perception, potentially reducing cravings for sugary or calorie-dense foods. This raises the possibility of appetite-regulating treatments that work by subtly adjusting how flavors are perceived, rather than suppressing hunger directly.
TRPM5 and Gut Immune Defense
TRPM5 is also active in specialized gut cells that detect nutrients and communicate with the immune system. These cells help coordinate immune responses and maintain a healthy balance between tolerance and defense.
Being able to inhibit or modulate TRPM5 in the gut could lead to new ways to control inflammation or regulate immune signaling, especially in conditions linked to chronic gut inflammation.
How the Discovery Was Made
To uncover this hidden control mechanism, researchers used a combination of cryo-electron microscopy (cryo-EM) and electrophysiology.
Cryo-EM allowed the team to visualize TRPM5 at near-atomic resolution, capturing detailed structural snapshots of the protein in different states. Electrophysiology experiments then confirmed how specific molecules affected the channel’s activity in real time.
This integrated approach revealed not only the existence of the pocket but also how different molecules reshape the protein to produce opposite functional outcomes.
Building on Earlier TRPM5 Research
This discovery builds on earlier work by the same research team. In 2021, they published the first high-resolution structural images of TRPM5, identifying potential regions that could serve as drug targets.
The current study takes that foundation further by demonstrating exactly how one of those regions operates as a unified control hub. Together, these findings provide a clear structural and functional blueprint for designing drugs that precisely tune TRPM5 activity.
Why This Discovery Matters
The identification of a single allosteric site that merges activation and inhibition reshapes how scientists think about ion channel regulation. Instead of separate on-switches and off-switches, TRPM5 uses one adaptable pocket that responds differently depending on molecular context.
This insight has broad implications beyond TRPM5 alone. It suggests that other ion channels may also contain hidden multifunctional control sites, waiting to be discovered.
Research Reference
Ruan Z et al., A single allosteric site merges activation, modulation and inhibition in TRPM5, Nature Chemical Biology (2026)
https://www.nature.com/articles/s41589-025-02097-7