Scientists Map the Development of Pancreatic Transport Channels That Deliver Digestive Enzymes
Organs in the human body are not just solid masses of cells. Many of them contain fluid-filled spaces called lumens, which play a crucial role in how organs function. These lumens act as internal transport systems, allowing fluids, enzymes, and other substances to move where they are needed. In the pancreas, lumens form an intricate ductal network that carries digestive enzymes into the small intestine. A new study has now shed light on how these pancreatic transport channels form, change shape, and organize themselves during development.
Understanding this process is important not only for basic biology but also for medicine. Problems in pancreatic duct formation are linked to several diseases, including cystic disorders and other duct-related abnormalities. Until now, however, scientists had only a limited understanding of how complex lumen shapes emerge, especially compared to the simpler spherical lumens often studied in lab models.
Why Pancreatic Lumens Matter
The pancreas has two main functions: producing hormones like insulin and releasing digestive enzymes that help break down food. These enzymes are made by exocrine cells and must travel through a branching duct system before reaching the intestine. The ducts themselves are formed by epithelial cells that surround lumens, creating hollow channels for transport.
If these lumens fail to develop properly, digestive enzymes may not reach their destination efficiently, or pressure can build up inside the ducts. This is why studying lumen formation is essential for understanding both normal organ development and pancreatic disease.
Moving Beyond Simple Lumen Models
For many years, research on lumen formation focused on simple systems that produce single, spherical lumens. While useful, these models do not reflect the reality of organs like the pancreas, which contain narrow, interconnected, and highly branched ducts.
To overcome this limitation, scientists turned to pancreatic organoids. These are three-dimensional structures grown in the lab from pancreatic cells that closely mimic real pancreatic tissue. Unlike simpler models, organoids can form a wide range of lumen shapes, from large hollow spheres to complex networks of thin tubes.
An International Research Collaboration
The new study was led by researchers from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, Germany. The work was carried out by the research group of Anne Grapin-Botton, in collaboration with scientists from the University of Tokyo, Academia Sinica in Taiwan, and the Institut de Génétique et de Biologie Moléculaire et Cellulaire in France.
By combining experimental organoid studies with computational modeling, the team set out to understand what controls lumen shape in developing pancreatic tissue.
Key Factors That Control Lumen Shape
The researchers identified three main factors that determine how pancreatic lumens form and what shape they take:
Cell proliferation rate
This refers to how fast the epithelial cells surrounding the lumen divide and multiply. When cells proliferate quickly, they can push and reshape the lumen structure.
Pressure inside the lumen
Fluid pressure builds up inside lumens as cells secrete fluid. High pressure tends to favor simpler, rounder shapes, while lower pressure allows lumens to stretch and branch.
Permeability of the surrounding cells
If epithelial cells are more permeable, fluid can leak out of the lumen more easily. This reduces internal pressure and supports the formation of narrow, interconnected channels.
The study showed that different combinations of these three factors lead to distinct lumen morphologies, ranging from large spherical cavities to star-shaped and branching networks that closely resemble real pancreatic ducts.
Manipulating Lumen Shape in the Lab
One of the most interesting aspects of the study is that the researchers were able to actively control lumen shape. By adding specific chemical compounds to the organoid culture medium, they altered cell proliferation rates and fluid pressure within the lumens.
They also changed how permeable the epithelial cells were. When permeability increased, pressure dropped, and lumens transitioned from spherical shapes to complex, branching networks. This demonstrated that lumen architecture is not fixed but can shift depending on physical and biological conditions.
The Role of Computational Modeling
Alongside experiments, the team developed a mathematical model to simulate how cells grow, divide, and interact with lumens over time. This model allowed the researchers to predict when and how transitions between different lumen shapes would occur.
Importantly, the modeling did not exist in isolation. Predictions from the model were fed back into experiments, helping scientists refine their understanding and confirm which parameters were most important. This feedback loop between theory and experiment was key to the study’s success.
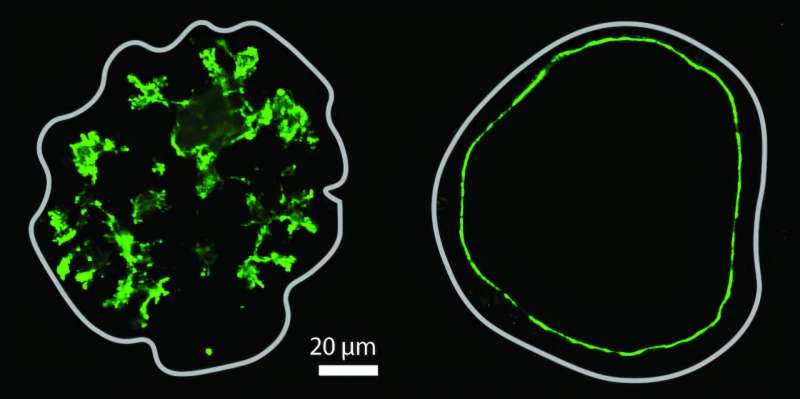
Star-Shaped Lumens and Real Pancreatic Tissue
One striking result was the formation of star-shaped lumens in pancreatic organoids. These structures closely resemble lumens observed in real developing pancreatic tissue. This finding suggests that organoids are not just convenient lab tools but highly accurate models for studying organ development.
The researchers also observed that early pancreatic tissue tends to be more permeable, naturally keeping lumen pressure low. As development progresses, epithelial cells become less permeable, pressure increases, and ducts mature into stable transport channels.
Implications for Pancreatic Diseases
The findings have important implications for understanding diseases that affect duct systems. In conditions where permeability, pressure, or cell proliferation are disrupted, lumens may form incorrectly. This could help explain cystic diseases, where ducts become abnormally enlarged or misshapen.
Because the model can predict how lumens respond to changes in physical conditions, it may eventually be used to test drugs or explore therapeutic strategies aimed at restoring healthy duct architecture.
Relevance Beyond the Pancreas
While the study focuses on the pancreas, its conclusions extend far beyond this single organ. Many organs, including the kidneys, lungs, and various glands, rely on narrow, branching ducts to function properly. The same basic principles of pressure, permeability, and cell growth may apply across these systems.
This makes the research highly relevant to the broader fields of organ development, tissue engineering, and regenerative medicine.
Why Organoid Research Is So Powerful
Organoids have become one of the most important tools in modern biology. They allow researchers to study complex developmental processes in a controlled environment, something that is often impossible in living organisms.
In this study, organoids made it possible to observe lumen formation in real time, manipulate key variables, and directly compare experimental results with computational predictions. This approach represents a major step forward in understanding how physical forces shape biological structures.
A Clear Framework for Lumen Morphogenesis
By identifying three core parameters that govern lumen shape, the researchers have provided a clear and testable framework for studying duct formation. Rather than treating lumen morphology as a mysterious outcome of development, the study shows it can be explained through measurable and controllable factors.
This clarity opens the door to future research that could explore how genetic mutations, environmental factors, or disease states interfere with these parameters.
Looking Ahead
The researchers emphasize that their model system can be expanded and refined. Future work could examine how additional factors, such as cell signaling pathways or mechanical constraints from surrounding tissue, influence lumen formation.
Ultimately, this research brings scientists closer to understanding not just how pancreatic ducts form, but why they take the shapes they do—and how those shapes are essential for life-sustaining functions like digestion.
Research paper:
https://www.nature.com/articles/s41556-025-01832-5