Protein Rac1 Plays Dual Roles in Repairing Damaged Kidneys, New Study Reveals
The kidneys quietly perform some of the most demanding jobs in the human body. Every day, they filter blood, remove waste, and carefully reclaim vital substances like water, glucose, ions, and small molecules that the body cannot afford to lose. A key player in this process is the proximal tubule, a highly active part of the kidney that depends heavily on energy to function properly. Because of this high workload, it is also especially vulnerable to injury.
A new study from researchers at Vanderbilt University Medical Center has uncovered an important and unexpected role for a protein called Rac1 in helping damaged kidneys repair themselves. The findings, published in the Proceedings of the National Academy of Sciences (PNAS) in 2025, show that Rac1 acts as a kind of molecular switch, coordinating both cell structure and energy recovery after injury.
Why the Proximal Tubule Is So Vulnerable to Damage
The proximal tubule is responsible for reabsorbing most of the filtered contents that pass through the kidney. This includes glucose, electrolytes, amino acids, and large volumes of water. All of this reabsorption requires enormous amounts of energy, which is why proximal tubule cells are packed with mitochondria.
However, this energy demand also creates a weakness. When blood flow to the kidney is reduced—a condition known as ischemia—these cells are among the first to suffer. Poor circulation damages mitochondria, disrupts energy production, and can cause the tubule cells to shrink or lose function. While the proximal tubule does have the ability to repair itself, the mechanisms behind this repair have not been fully understood.
Rac1 Emerges as a Key Regulator of Kidney Repair
The new study identifies Rac1, a small GTPase protein best known for regulating the actin cytoskeleton, as a central figure in proximal tubule repair. The actin cytoskeleton is a dynamic internal framework that gives cells their shape and allows components inside the cell to move and reorganize.
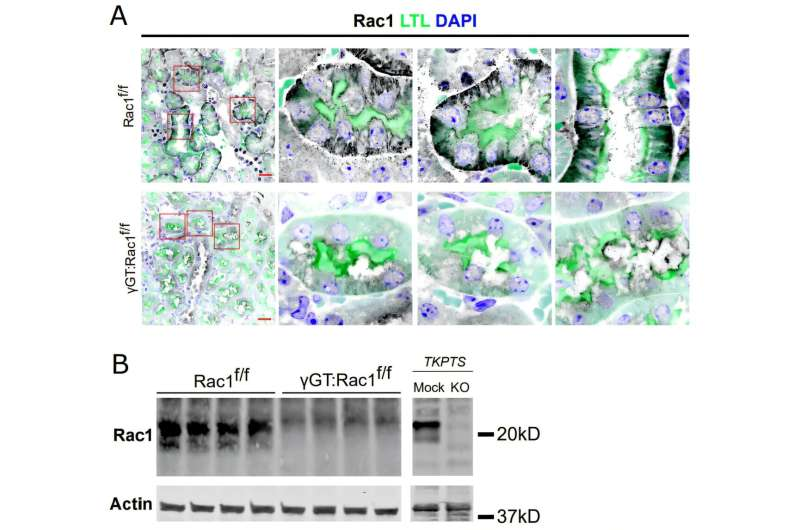
Researchers found that Rac1 does much more than simply maintain cell structure. In injured proximal tubule cells, Rac1 promotes the formation and organization of actin filaments, which in turn enables damaged mitochondria to be removed and replaced with healthy ones. This process restores mitochondrial respiration, improves ATP production, and allows the tubule cells to regain their normal function.
Without Rac1, this repair process breaks down. Damaged mitochondria accumulate instead of being cleared, energy production declines, and the cells gradually become atrophic, meaning they lose size and functionality.
How Rac1 Connects Cell Structure and Energy Production
One of the most interesting aspects of the study is how it links two systems that are often studied separately: cell architecture and metabolism.
Mitochondria are responsible for converting nutrients into ATP through a process called respiration. The actin cytoskeleton, on the other hand, is usually thought of as a structural and transport network. This research shows that Rac1 couples these two systems, ensuring that mitochondrial quality control depends on an organized and responsive cytoskeleton.
When ischemic injury damages mitochondria, Rac1-driven actin remodeling helps the cell identify, remove, and replace those faulty organelles. This coordinated response highlights how cell structure actively participates in metabolic recovery, rather than serving as a passive scaffold.
Building on Earlier Rac1 Research
This discovery did not come out of nowhere. The same research group, led by Fabian Bock, along with Roy Zent and colleagues, has been studying Rac1 in kidney biology for several years.
Earlier work showed that Rac1 is essential for maintaining the integrity and function of epithelial cells lining the collecting ducts, which are located downstream from the proximal tubule. In previous studies, the team demonstrated that Rac1 helps collecting ducts recover from injury caused by obstructed urinary flow.
The current findings extend Rac1’s importance upstream to the proximal tubule, showing that the protein plays multiple, context-dependent roles across different parts of the kidney. This dual role strengthens the idea that Rac1 is a master regulator of kidney epithelial health.
Why Mitochondria Matter So Much in Kidney Health
Kidney cells, especially those in the proximal tubule, have very limited metabolic flexibility. Unlike some other cell types, they cannot easily switch to alternative energy sources when mitochondria fail. This makes mitochondrial health absolutely critical.
If mitochondria stop functioning properly, proximal tubule cells quickly lose their ability to reabsorb essential molecules. Over time, this contributes to kidney dysfunction and can worsen conditions such as acute kidney injury and potentially chronic kidney disease.
By showing that Rac1 directly supports mitochondrial turnover and function, the study provides new insight into why some kidneys recover well after injury while others do not.
Implications for Future Kidney Therapies
One of the most exciting outcomes of this research is its therapeutic potential. If Rac1-dependent pathways can be safely targeted, it may be possible to enhance kidney repair after severe or widespread injury.
Rather than simply preventing damage, future treatments could focus on actively promoting recovery, especially in patients who experience ischemic injury during surgery, transplantation, or severe illness. Targeting the molecular pathways that control actin organization and mitochondrial quality could open new avenues for regenerative therapies in nephrology.
That said, Rac1 is involved in many cellular processes throughout the body, so any therapeutic approach would need to be carefully controlled to avoid unwanted side effects.
A Broader Look at Rac1 Beyond the Kidney
Outside the kidney, Rac1 has been studied for its roles in cell migration, immune responses, and development. It is also involved in regulating oxidative stress and maintaining cellular balance in other organs.
Some emerging research suggests that Rac1 activity may change in metabolic diseases, including diabetes, which is a major risk factor for kidney damage. Understanding how Rac1 functions across different tissues could help scientists better grasp its broader role in human health and disease.
What This Study Adds to Kidney Science
This research adds a critical piece to the puzzle of kidney repair by showing that structural proteins and energy systems are deeply interconnected. It challenges the idea that cytoskeletal proteins are only about shape and stability, revealing instead that they play active roles in organelle health and metabolic recovery.
Most importantly, it highlights the proximal tubule as a cell type that is both highly specialized and highly vulnerable, relying on precise molecular coordination to survive injury.
Research Reference
Rac1 promotes proximal tubule kidney repair by coupling the actin cytoskeleton to mitochondrial function
Proceedings of the National Academy of Sciences (2025)
https://www.pnas.org/doi/10.1073/pnas.2504565122