Mapping Gene Disruptions in Sporadic Early-Onset Alzheimer’s Disease Across Key Brain Regions
Researchers at UTHealth Houston have taken a major step toward understanding one of the most puzzling and aggressive forms of Alzheimer’s disease. In a new study published in Science Advances, scientists closely examined how genes behave and are regulated in the brains of people with sporadic early-onset Alzheimer’s disease (sEOAD), uncovering widespread disruptions across multiple brain regions at an unprecedented level of detail.
Alzheimer’s disease is most commonly associated with older adults, but around 5% to 10% of patients develop symptoms before the age of 65. These early-onset cases are often more aggressive. A small fraction of them are linked to inherited mutations in well-known genes such as APP, PSEN1, and PSEN2. However, about 90% of early-onset cases are classified as sporadic, meaning they occur without these known genetic mutations. This large group has remained poorly understood, largely because its genetic and molecular roots are unclear.
The new study focuses specifically on this understudied population, aiming to answer a key question: what goes wrong at the molecular level in the brains of people with sporadic early-onset Alzheimer’s disease?
Looking Beyond a Single Brain Region
One of the most important aspects of this research is its scope. Previous studies of Alzheimer’s disease, especially those looking at gene activity, have typically focused on a single brain region, most often the prefrontal cortex. While this region is important, Alzheimer’s disease affects multiple interconnected areas of the brain, each with its own role in memory, cognition, and behavior.
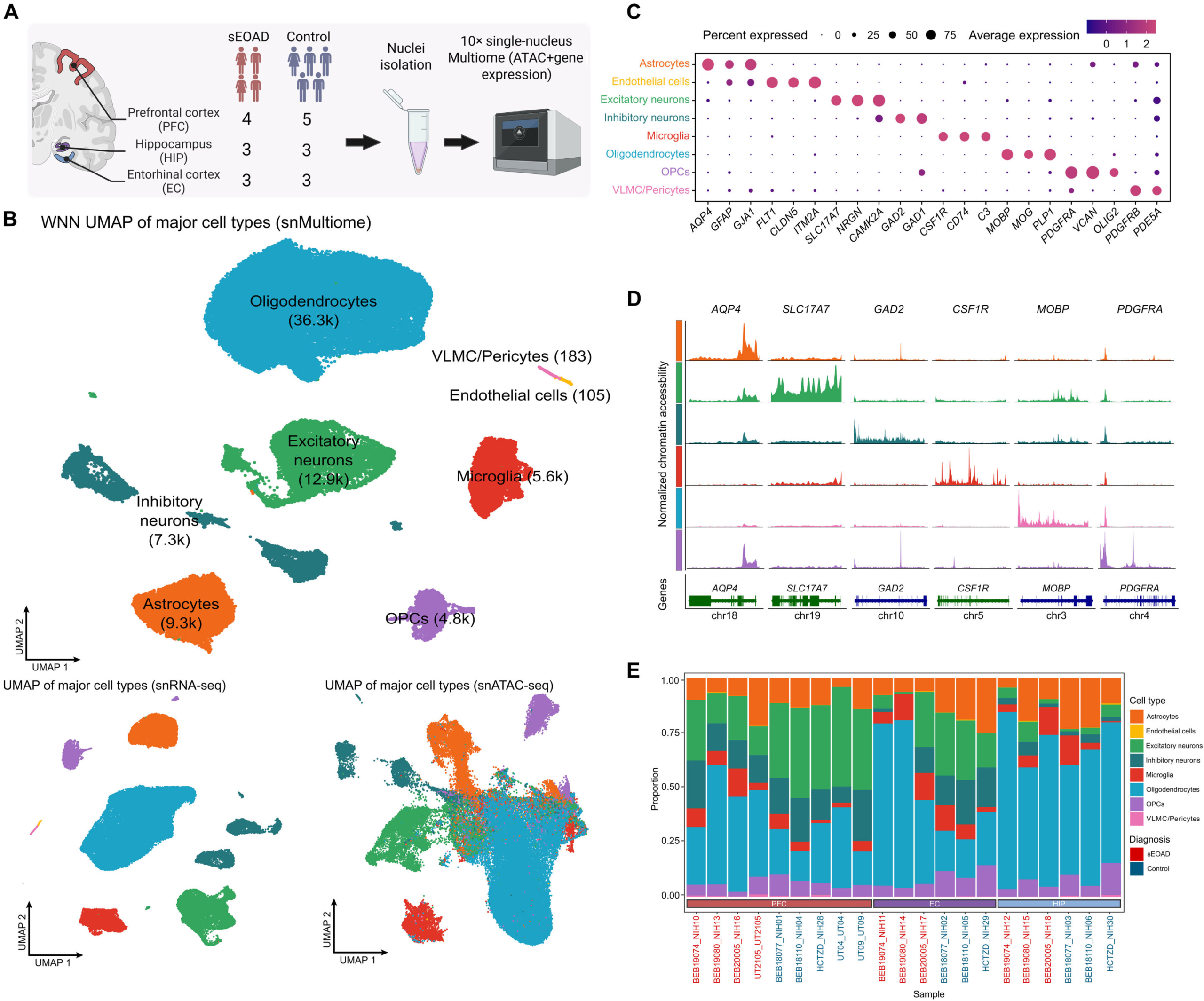
In this study, researchers analyzed three critical brain regions that are deeply involved in Alzheimer’s disease progression:
- The prefrontal cortex, which is essential for decision-making and complex thinking
- The entorhinal cortex, a key gateway for memory and navigation
- The hippocampus, central to learning and memory formation
By examining all three regions side by side, the researchers were able to identify both shared disruptions and region-specific changes, something that had not been done before in the context of sporadic early-onset Alzheimer’s disease.
Advanced Single-Nucleus Multiomics at Work
To achieve this level of insight, the team used an advanced technique known as single-nucleus multiomics. Instead of studying whole cells, this approach isolates the nucleus of each cell, which acts as the control center for gene activity. This allows scientists to examine multiple layers of biological information at once.
Specifically, the researchers looked at:
- Gene expression, which genes are turned on or off
- Gene regulation, how DNA accessibility and regulatory elements control that expression
Using this technique, the team analyzed more than 76,000 individual nuclei collected from the three brain regions. This massive dataset allowed them to study gene behavior at the level of individual cell types, including neurons, immune cells, and various types of support cells.
Where the Most Severe Disruptions Occur
One of the clearest findings from the study is that not all brain regions are affected equally. While changes were seen across all three areas, the entorhinal cortex and hippocampus showed the most severe disruptions in gene regulation and expression.
This aligns closely with what clinicians observe in patients. These two regions are among the earliest and most heavily affected areas in Alzheimer’s disease, particularly when it comes to memory loss and spatial disorientation. The molecular evidence from this study reinforces the idea that these regions are especially vulnerable in sporadic early-onset Alzheimer’s disease.
Key Gene “Switches” That Go Wrong
The researchers identified disruptions in genes that act like molecular switches, controlling how cells behave and respond to their environment. Two of these switches stood out:
- RFX4, primarily affected in astrocytes, a type of support cell in the brain
- IKZF1, disrupted in microglia, the brain’s immune cells
Astrocytes play a critical role in maintaining brain health, regulating inflammation, and supporting neurons. Microglia, on the other hand, are responsible for immune defense and cleanup in the brain. When functioning properly, both cell types protect neural tissue. When their regulatory switches malfunction, they can contribute to damage instead.
The study found that disruptions in RFX4 and IKZF1 affected pathways involved in neuroinflammation and synaptic communication, the specialized junctions where neurons send electrical and chemical signals to one another. These pathways are essential for healthy brain function and are known to be heavily impacted in Alzheimer’s disease.
The Role of Glial Cells and Inflammation
Beyond neurons, the study highlights the importance of glial cells, a broad group of support cells that includes astrocytes and microglia. These cells are increasingly recognized as major players in neurodegenerative diseases.
Astrocytes normally help protect the brain by responding to injury and infection. However, in the presence of amyloid plaques, a hallmark of Alzheimer’s disease, astrocytes may become overactive. Instead of helping, they can trigger excessive inflammation that damages nearby neurons.
Microglia also showed altered regulatory patterns, suggesting changes in how the brain’s immune system responds to disease-related threats. Together, these findings point to immune dysregulation as a central feature of sporadic early-onset Alzheimer’s disease.
Similarities and Differences With Other Brain Disorders
Interestingly, the study found that while some molecular changes in sporadic early-onset Alzheimer’s disease overlap with those seen in late-onset Alzheimer’s, others appear to be unique. Even more surprising was the discovery of shared genetic vulnerabilities with certain psychiatric conditions, including schizophrenia and bipolar disorder.
This does not mean these conditions are the same, but it does suggest that some underlying regulatory pathways may be disrupted across different brain disorders. These overlaps could help explain why certain symptoms or risk factors appear across multiple neurological and psychiatric diseases.
Why This Study Matters
This research represents a major advance for several reasons:
- It is the first study to map gene expression and regulation across three brain regions in sporadic early-onset Alzheimer’s disease at single-cell resolution
- It provides a detailed molecular atlas that other researchers can use to explore disease mechanisms
- It identifies specific regulatory genes that could become targets for future therapies
By understanding exactly how gene regulation breaks down in specific cell types and brain regions, scientists move closer to precision medicine approaches. Instead of treating Alzheimer’s disease as a single condition, future therapies could target the specific molecular disruptions present in different patient subgroups.
Expanding the Bigger Picture of Alzheimer’s Disease
Alzheimer’s disease is often described as devastating not only because it affects memory, but because it gradually alters personality, independence, and identity. Studies like this help shift the conversation from symptoms to mechanisms, offering hope that deeper understanding will eventually lead to better treatments.
Sporadic early-onset Alzheimer’s disease, in particular, has long existed in a gray area between genetic and environmental explanations. By showing how gene regulation is disrupted even in the absence of known mutations, this research fills an important gap and opens new directions for investigation.
Research Paper:
https://www.science.org/doi/10.1126/sciadv.adw4917