Implant Provides Lasting Relief for Treatment-Resistant Depression, Study Finds
Major depression is far more common than many people realize. Around 20% of U.S. adults experience it at some point in their lives. For most, symptoms ease after trying a few medications or therapy approaches. But for a significant group—up to one-third of patients—standard treatments simply don’t work. This condition is known as treatment-resistant depression, and it often lasts for decades, severely limiting daily life.
A new large-scale study offers hopeful news for this group. Researchers have found that a small implanted device that stimulates the vagus nerve can provide substantial and long-lasting relief for people with the most severe, long-standing forms of depression. Even more striking, many patients continued to benefit for at least two years, a rarity in this population.
The study was led by researchers at Washington University School of Medicine in St. Louis and conducted across dozens of medical centers in the U.S. The findings come from an ongoing clinical trial called RECOVER, and the results were published in the International Journal of Neuropsychopharmacology.
Who the Study Focused On
This trial did not involve people with mild or moderate depression. It focused on patients with extreme treatment resistance—arguably some of the most difficult cases in psychiatry.
On average, participants had:
- Lived with depression for 29 years
- Tried 13 different treatments that failed
- Undergone advanced interventions such as electroconvulsive therapy (ECT) and transcranial magnetic stimulation (TMS)
- Severe functional impairment, with three-quarters unable to work
In short, this was a group with very few remaining options.
How the Implant Works
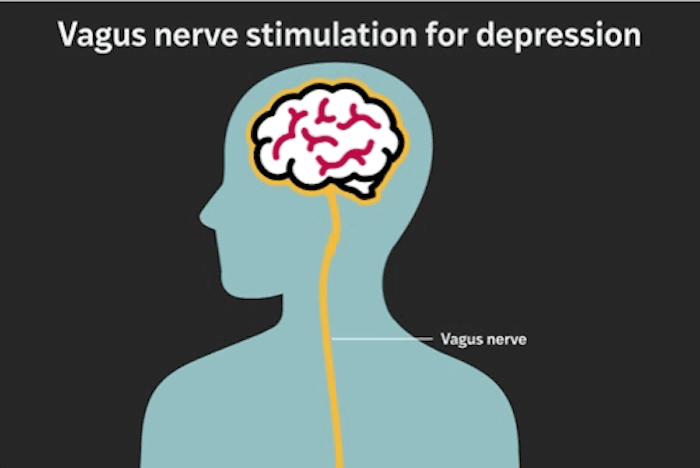
The therapy studied is called vagus nerve stimulation (VNS). It involves surgically implanting a device under the skin in the chest. From there, the device sends carefully calibrated electrical pulses to the left vagus nerve, one of the body’s major communication pathways between the brain and internal organs.
The device used in the study, known as the VNS Therapy System, is manufactured by LivaNova U.S., Inc. While VNS has been approved for treatment-resistant depression for years, its use has been limited due to cost and inconsistent insurance coverage.
One of the main goals of the RECOVER trial is to generate strong, long-term data that could influence coverage decisions by the U.S. Centers for Medicare and Medicaid Services (CMS). If CMS approves broader coverage, many private insurers are likely to follow.
How the RECOVER Trial Was Designed
The RECOVER trial enrolled nearly 500 patients at 84 clinical sites across the U.S. Every participant received an implanted VNS device, but for scientific rigor, only half of the devices were activated during the first year. This allowed researchers to compare outcomes between active treatment and inactive (control) groups.
Researchers tracked several outcomes:
- Depression symptom severity
- Quality of life
- Daily functioning
A response was considered meaningful if symptoms improved by at least 30% from baseline. A response was considered substantial if symptoms decreased by 50% or more.
What Happened After One Year
Earlier results from the first, blinded year of the trial showed that patients with activated devices spent more time feeling better, with improvements in mood, daily function, and quality of life compared to those whose devices were inactive.
However, one commonly used depression rating scale—the Montgomery-Åsberg Depression Rating Scale—did not show a statistically significant difference between the two groups during that first year. While this raised questions initially, researchers emphasized that symptom scales alone don’t capture the full picture of how patients live and function.
Two-Year Results Show Lasting Benefits
The newly published analysis focused on patients who had active VNS therapy from the very start of the trial. Researchers wanted to see whether benefits seen at 12 months would last through 24 months, and whether some patients might improve more slowly over time.
Here’s what they found:
- Out of 214 patients receiving active treatment from the beginning, 69% (147 people) showed a meaningful response at one year in at least one key measure.
- Among those who responded at one year, over 80% maintained or improved their benefits at the two-year mark across all outcomes.
- For patients with a substantial response at one year, 92% were still benefiting after two years.
- Nearly one-third of patients who did not improve after the first year showed meaningful benefits by the end of the second year.
This suggests that VNS may work slowly for some individuals, and patience may be critical when evaluating its effectiveness.
Low Relapse and Remarkable Remission Rates
Another standout finding was the low relapse rate among people who benefited from the treatment, especially those with stronger early responses.
Perhaps most impressive, more than 20% of treated participants—39 people—were in full remission after two years. In practical terms, this means their symptoms improved enough that they could function normally in daily life.
For patients who had lived with severe depression for decades, this level of recovery is highly unusual.
Why These Results Matter
Most studies involving people with severe treatment-resistant depression show poor durability, with benefits fading over time. In contrast, this trial showed people getting better and staying better, even after years of illness.
For clinicians, the findings suggest:
- Even partial improvement can be life-changing in severe cases
- Long-term follow-up is essential when evaluating treatments like VNS
- Lack of response at one year doesn’t necessarily mean failure
For patients and families, the results offer realistic hope—not a miracle cure, but a meaningful option when nearly everything else has failed.
A Closer Look at Vagus Nerve Stimulation
The vagus nerve plays a role in mood regulation, inflammation, heart rate, digestion, and stress response. Stimulating it appears to influence brain regions involved in emotion and motivation.
VNS has already been used for epilepsy for decades, which provided much of the safety data that allowed researchers to explore its use in depression. Side effects are generally related to stimulation itself, such as voice changes or throat sensations, rather than systemic drug effects.
Unlike medications, VNS does not rely on daily dosing and does not interact with other psychiatric drugs, making it a unique addition rather than a replacement.
What Comes Next
The RECOVER trial is still ongoing, and researchers continue to collect long-term data. The hope is that these findings will help CMS and private insurers reconsider coverage, making the therapy accessible to more patients who currently cannot afford it.
While VNS is not for everyone and requires surgery, this study strongly suggests it may be one of the most durable treatment options available for people with the most severe, treatment-resistant depression.
Research Paper:
Durability of the benefit of vagus nerve stimulation in markedly treatment-resistant major depression: a RECOVER trial report
International Journal of Neuropsychopharmacology (2026)
https://academic.oup.com/ijnp/article/29/1/pyaf080/8423597