New Mouse Models Advance the Study of a Crucial Male Fertility Gene

Researchers at the University of Hawaiʻi at Mānoa have developed new mouse models that significantly improve scientists’ ability to study a gene that plays a central role in male fertility. This work focuses on a Y-chromosome gene called ZFY, which has long been known to be essential for normal sperm development but has remained difficult to study at the protein level. By combining years of prior research with modern genome-editing techniques, the team has now created tools that could deepen understanding of male infertility and eventually support better diagnostic or treatment strategies.
The study was led by Professor Monika Ward from the John A. Burns School of Medicine and the Yanagimachi Institute for Biogenesis Research, and it was published in the journal BMC Genomics. At its core, the research addresses a long-standing challenge in reproductive biology: understanding exactly how ZFY works inside male germ cells and which genes it controls during spermatogenesis.
Why the ZFY Gene Matters for Male Fertility
The ZFY gene is located on the Y chromosome, meaning it is present only in males. In mice, this gene exists in two closely related versions known as Zfy1 and Zfy2. These genes produce proteins that are believed to act as transcription factors, molecules that regulate other genes by switching them on or off.
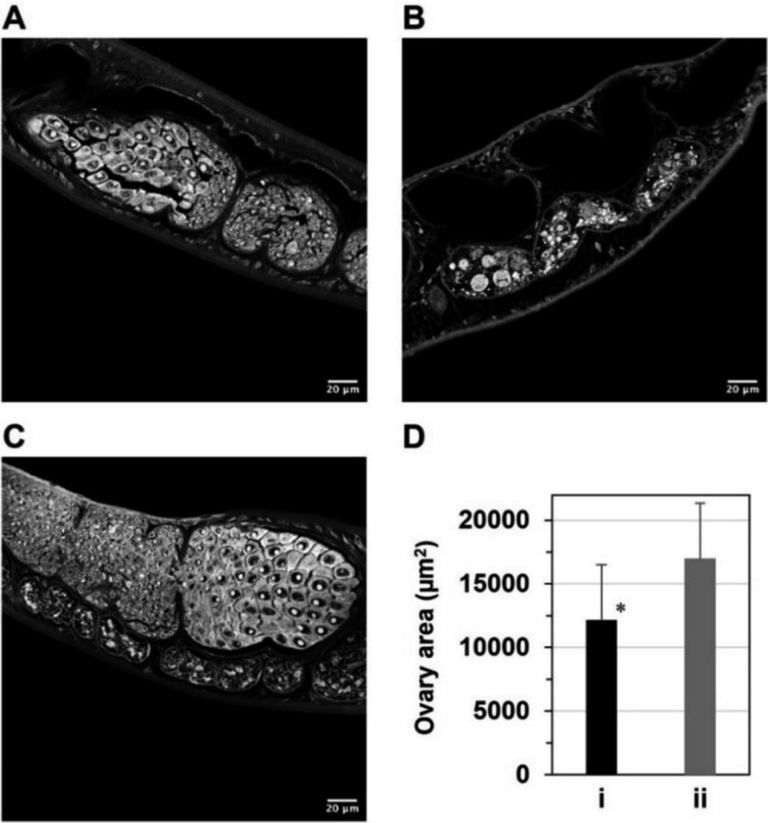
Earlier studies from the same research group showed just how critical ZFY is. When male mice lacked both copies of Zfy1 and Zfy2, they were found to be completely infertile. Their testes showed widespread disruption of genes involved in sperm development, cell survival, and other processes essential for producing functional sperm. These findings firmly established ZFY as a key player in male reproduction.
However, despite knowing that ZFY was important, researchers faced a major limitation: they could not reliably detect or track ZFY proteins inside cells. Without being able to see where and when these proteins are produced, it was impossible to fully understand their role.
Using CRISPR to Create New Research Tools
To overcome this barrier, the research team turned to CRISPR–Cas9 genome editing, a powerful technique that allows scientists to make precise changes to DNA. In this study, CRISPR was used to insert small molecular “tags” directly into the natural locations of the Zfy1 and Zfy2 genes in mice.
These tags act like molecular labels. They do not disrupt the normal function of the genes, but they make it possible for researchers to detect, isolate, and analyze the ZFY proteins using standard laboratory techniques. This approach is known as a knock-in strategy, because the tags are added without removing or disabling the original gene.
With these newly engineered mouse models, scientists can now study ZFY in ways that were not previously possible.
Tracking Where and When ZFY Proteins Are Made
One of the most important outcomes of the study is that researchers were able, for the first time, to determine which male germ cells produce ZFY1 and ZFY2 proteins, and at what levels. Male germ cells go through several distinct stages as they develop into mature sperm, and gene activity can vary dramatically across these stages.
Using the tagged mouse models, the team identified specific cell types within the testes that express each ZFY protein. They were also able to compare the relative abundance of ZFY1 versus ZFY2, shedding light on how these two closely related proteins might have overlapping or distinct roles during spermatogenesis.
This level of detail had been out of reach in earlier studies because existing antibodies could not reliably distinguish between the two proteins or detect them with sufficient sensitivity.
Understanding ZFY as a Gene Regulator
ZFY is thought to function primarily as a regulatory protein, influencing the activity of many other genes. The new mouse models open the door to identifying exactly which genes ZFY regulates and how those genes contribute to sperm development and survival.
With the ability to isolate ZFY proteins directly, researchers can now perform experiments to map their DNA-binding sites, identify protein interaction partners, and connect ZFY activity to changes in gene expression. This is a crucial step toward understanding the molecular networks that govern male fertility.
Over time, these insights could help explain why disruptions in Y-chromosome genes sometimes lead to infertility, even when other aspects of reproductive anatomy appear normal.
Broader Implications for Male Infertility Research
Male infertility affects a significant number of couples worldwide, yet in many cases, the underlying genetic causes remain unclear. Y-chromosome genes like ZFY are especially important because they are unique to males and are often overlooked in standard genetic analyses.
The new mouse models provide a foundational research tool that can be used by scientists studying not only basic reproductive biology but also the genetic basis of infertility. By clarifying how ZFY supports spermatogenesis, researchers may eventually be able to connect specific genetic defects to clinical outcomes.
While this study does not propose immediate treatments, it lays the groundwork for future research that could improve diagnosis, risk assessment, or even therapeutic strategies related to male infertility.
A Closer Look at Spermatogenesis
To appreciate why genes like ZFY are so important, it helps to understand spermatogenesis, the process by which sperm are produced. This process involves multiple stages, including mitotic division of germ cells, meiosis to reduce chromosome number, and final maturation into sperm capable of fertilization.
Each stage is tightly regulated by hundreds of genes. A disruption at any point can lead to reduced sperm count, abnormal sperm shape, or complete infertility. Transcription factors like ZFY help coordinate these stages by ensuring the right genes are active at the right time.
Because spermatogenesis is both complex and highly specialized, even small changes in gene regulation can have large effects on fertility.
Why Mouse Models Are Still Essential
Although human studies are critical for understanding infertility, mouse models remain one of the most powerful tools in reproductive biology. Mice share many genetic and physiological similarities with humans, and their reproductive systems are well studied.
The ability to precisely modify mouse genes using CRISPR allows researchers to test hypotheses that would not be possible or ethical in humans. Insights gained from mouse studies often guide human research and help identify genes worth investigating in clinical settings.
The ZFY-tagged mouse models represent a strong example of how carefully designed animal studies can answer long-standing biological questions.
Looking Ahead
The development of these new mouse models marks an important step forward in understanding how Y-chromosome genes support male fertility. By making ZFY proteins visible and measurable, researchers now have the tools needed to explore their function in depth.
As future studies build on this work, scientists may uncover new connections between gene regulation, sperm development, and infertility. While practical applications may still be years away, the ability to finally study ZFY at the protein level is a major advance for the field.
Research paper:
https://doi.org/10.1186/s12864-025-12462-4