Immune-Targeting Vaccine Shows Promise in Stopping Cancer Early in People With Lynch Syndrome
A new experimental cancer vaccine is offering fresh hope for people living with Lynch syndrome, a hereditary condition that dramatically increases the risk of developing several types of cancer. Researchers from The University of Texas MD Anderson Cancer Center have reported encouraging results from a Phase Ib/II clinical trial of an investigational vaccine called NOUS-209, suggesting it may help the immune system detect and eliminate cancerous changes before full-blown tumors develop.
The study, published in Nature Medicine in January 2026, focuses on the idea of cancer interception—intervening at the earliest stages of disease, even before cancer becomes clinically apparent. For individuals with Lynch syndrome, who often face repeated screenings or life-altering preventive surgeries, this approach could represent a meaningful shift in how cancer risk is managed.
Understanding Lynch Syndrome and Cancer Risk
Lynch syndrome is caused by inherited mutations in mismatch repair (MMR) genes, which normally correct DNA errors during cell division. When these genes don’t function properly, DNA mistakes accumulate, leading to a phenomenon known as microsatellite instability (MSI). As a result, people with Lynch syndrome are much more likely to develop cancers—particularly colorectal, endometrial, urothelial, and several other cancer types—often at a younger age than the general population.
Because of this elevated risk, current medical management relies heavily on frequent surveillance, such as regular colonoscopies, or preventive surgeries that remove at-risk organs before cancer has a chance to form. While these strategies can be effective, they are also physically and emotionally demanding and can significantly affect quality of life. This is where immune-based preventive approaches like NOUS-209 come into the picture.
What Is the NOUS-209 Vaccine?
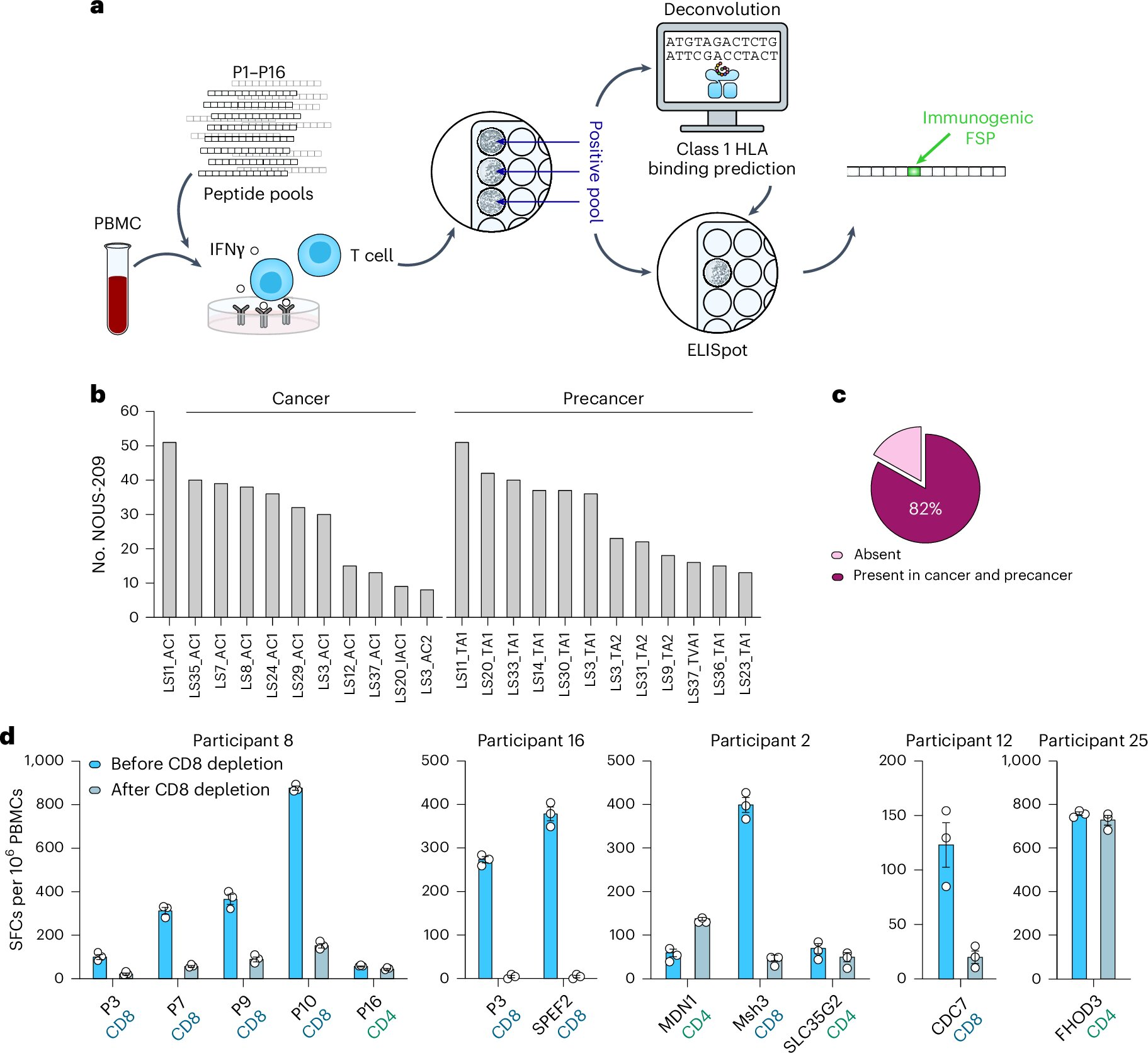
NOUS-209 is an experimental neoantigen-based cancer vaccine designed specifically for people with Lynch syndrome. Rather than targeting a single tumor type, the vaccine focuses on shared molecular signals that appear very early in cancer development.
In Lynch syndrome, faulty DNA repair leads to the production of abnormal proteins known as frameshift peptides (FSPs). These FSPs are present not only in established cancers but also in precancerous lesions, making them attractive targets for immune intervention. NOUS-209 introduces selected FSPs to the immune system in a controlled way, essentially training immune cells to recognize these abnormal markers as threats.
The goal is to activate CD8+ T cells, a type of immune cell capable of directly killing abnormal or malignant cells, before those cells can grow into invasive cancer.
Inside the Phase Ib/II Clinical Trial
The trial enrolled 45 individuals with Lynch syndrome and was designed primarily to evaluate safety and immunogenicity, rather than long-term cancer prevention outcomes. Participants received the NOUS-209 vaccine and were closely monitored for side effects, immune responses, and early signs of clinical impact.
One of the most important findings was that the vaccine was generally well tolerated. Researchers did not observe any serious adverse events related to the treatment, an essential requirement for any preventive therapy intended for otherwise healthy individuals.
From an immune standpoint, the results were particularly striking. All participants developed strong T-cell responses against the targeted cancer-related antigens. These immune responses did not fade quickly; instead, they showed signs of durability and immune memory, meaning the immune system retained its ability to recognize the targets over time.
Importantly, researchers found that annual retreatment further boosted immune responses, suggesting that the vaccine could potentially be administered on a recurring schedule to maintain protection.
Evidence of Cancer Interception
While the trial was not designed to definitively prove cancer prevention, researchers did observe encouraging early signals. One year after vaccination, participants showed fewer precancerous lesions, and notably, no new advanced polyps were detected during follow-up examinations.
These findings hint that NOUS-209 may help halt the progression from abnormal cells to more dangerous precancerous or cancerous growths. Although larger studies are needed to confirm this effect, the early data support the idea that immune-based interception could play a role in managing hereditary cancer risk.
Why This Approach Is Different
Most cancer vaccines to date have focused on treating existing tumors. NOUS-209 is different because it targets shared, early-stage molecular features seen across many Lynch-associated cancers. This makes it less personalized than some neoantigen therapies, but potentially far more scalable and practical for preventive use.
Another key advantage is timing. By training the immune system before cancer develops, this strategy aims to stay one step ahead of disease rather than reacting after tumors appear.
Broader Context: Cancer Prevention Through Immunology
The idea of using the immune system to prevent cancer is gaining traction across oncology research. Advances in immunotherapy, particularly checkpoint inhibitors, have already transformed cancer treatment. Researchers are now exploring how similar principles can be applied earlier in the disease process.
For high-risk populations like those with Lynch syndrome, preventive vaccines could eventually complement or even reduce the need for invasive procedures. However, experts stress that screening and standard preventive care remain essential, at least until long-term clinical benefits are clearly established.
Limitations and Next Steps
Despite the promising results, the study has important limitations. With just 45 participants, it is too small to draw firm conclusions about long-term cancer prevention. The trial was also not randomized or placebo-controlled, which means results must be interpreted cautiously.
Researchers are now working to understand how NOUS-209 performs in larger and higher-risk populations, how long immune protection lasts, and what the optimal dosing schedule might be over multiple years. These future studies will be critical in determining whether the vaccine can move beyond early-phase trials and into broader clinical use.
What This Could Mean for People With Lynch Syndrome
If future trials confirm these findings, NOUS-209 could represent a new preventive option for people living with Lynch syndrome—one that relies on immune education rather than constant surveillance or irreversible surgical decisions. While it is still early days, the concept of teaching the immune system to recognize cancer before it takes hold is an exciting development in preventive medicine.
For now, the results offer a strong scientific foundation and a glimpse into how immune-targeting vaccines might reshape cancer prevention in genetically high-risk groups.
Research paper:
https://www.nature.com/articles/s41591-025-04182-9