Scientists Discover a Bone “Switch” That Could Help Stop Osteoporosis
Researchers from Leipzig University in Germany have uncovered a hidden biological mechanism that may pave the way for new treatments against osteoporosis. They identified a receptor known as GPR133 that plays a crucial role in maintaining bone strength. Even more exciting, they tested a compound called AP503 that activates this receptor, boosting bone density and even reversing osteoporosis-like conditions in mice.
This discovery could be a game-changer for millions of people, especially women after menopause, who face bone loss as they age. Let’s go through the study, what exactly GPR133 is, how AP503 works, and why this matters for the future of bone health. Along the way, I’ll also add some background information about osteoporosis and GPCR biology so you have the full picture.
Understanding Osteoporosis and Why It Matters
Osteoporosis is a disease where bones become weak, brittle, and more likely to fracture. It’s often called the “silent disease” because bone loss occurs gradually and without obvious symptoms until a fracture happens.
- In Germany alone, about six million people suffer from osteoporosis, the majority of them women.
- Globally, the numbers are staggering — hundreds of millions are affected, with risks increasing sharply after menopause due to falling estrogen levels.
- Current medications for osteoporosis include bisphosphonates, denosumab, selective estrogen receptor modulators, and newer anabolic agents like teriparatide. While these can help, they come with limitations such as side effects, cost, or reduced effectiveness after long-term use.
That’s why researchers are constantly searching for new biological targets that can safely restore bone strength. GPR133 now emerges as one such promising target.
The Discovery of GPR133
GPR133, also known as ADGRD1, belongs to the large family of adhesion G protein-coupled receptors (aGPCRs). GPCRs are a huge group of proteins found on cell surfaces, and they are responsible for transmitting signals from the outside world into cells. Around one-third of all modern drugs already target GPCRs, but the adhesion type has remained relatively underexplored.
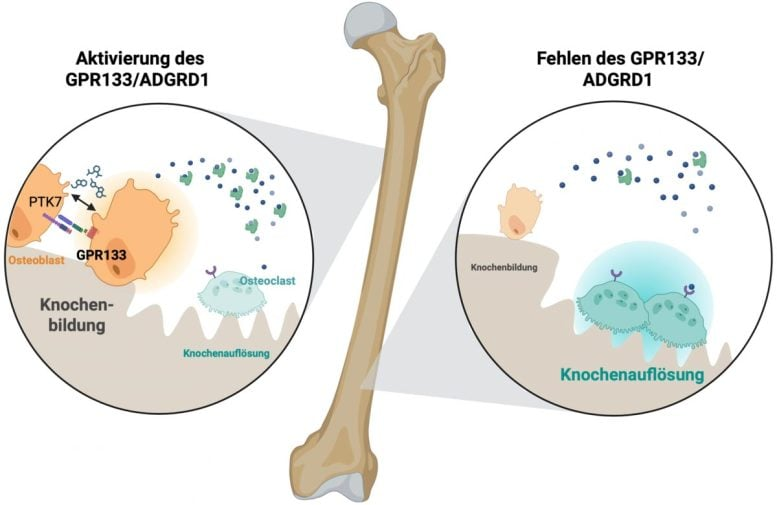
The Leipzig research team showed that GPR133 is essential for bone development and strength. When this receptor is missing or impaired, mice quickly lose bone density — developing signs similar to early-onset osteoporosis in humans.
The receptor is mechanosensitive, meaning it responds to physical forces like strain or loading. It also interacts with neighboring bone cells through signals, including a protein called PTK7. When switched on, GPR133 launches a chain reaction inside the bone:
- It stimulates osteoblasts, the cells that build new bone.
- It suppresses osteoclasts, the cells that break down bone.
This dual effect makes it particularly powerful. Bones are in constant turnover, and the balance between these two cell types determines whether bones are strong or fragile.
Enter AP503 – A Small Molecule with Big Effects
Through a computer-assisted screening process, the researchers identified AP503, a small molecule that specifically activates GPR133.
When they gave AP503 to mice, the results were striking:
- In healthy mice, AP503 significantly boosted bone strength.
- In mice with osteoporosis-like symptoms (including those that had undergone ovary removal to mimic menopause), AP503 actually reversed bone loss.
- The treatment improved bone density, thickness, and resistance to mechanical stress.
- It increased the number of osteoblasts and osteocytes while reducing the number of osteoclasts.
Even more importantly, AP503 only worked when GPR133 was present. In mice where the gene for this receptor had been deleted, AP503 had no effect. That confirms that the drug is indeed acting through this specific pathway.
How the Signaling Works
When GPR133 is activated, it triggers the Gs–cAMP pathway, a well-known signaling route inside cells. This activation leads to β-catenin signaling, which is central for bone growth and maintenance.
This process mimics what normally happens when bones are put under mechanical strain — like walking, running, or lifting weights. In other words, AP503 artificially reproduces the effect of exercise on bone cells. This opens the door to combining pharmacological treatment with physical activity for even stronger results.
Beyond Bones: Potential Benefits for Muscles
Interestingly, this isn’t the first time Leipzig researchers have looked into GPR133. In an earlier study, they found that activating this receptor with AP503 also strengthens skeletal muscle. That means a therapy targeting GPR133 could potentially tackle both bone and muscle loss at once.
For older adults, this combination is hugely valuable. Weak bones plus weak muscles form a dangerous cycle that leads to falls, fractures, and loss of independence. A treatment that addresses both together would represent a major breakthrough in geriatric medicine.
Why This Discovery Matters
- New Treatment Pathway: Current osteoporosis drugs often focus either on slowing bone breakdown or stimulating bone formation. GPR133 activation seems to do both.
- Mechanosensitivity: Because it responds to mechanical strain, it could work naturally with exercise — possibly leading to better, more durable outcomes.
- Wide Potential Use: Beyond osteoporosis, GPR133-based therapies might help in conditions of bone weakness, recovery after fractures, or even muscle-wasting diseases.
- Drug Development Opportunity: GPCRs are already proven drug targets. Pharmaceutical companies are very experienced in designing molecules for this receptor family, which could speed up translation into real therapies.
Important Limitations
While the findings are exciting, it’s important to highlight that all of this research so far has been done in mice and cell cultures.
- We don’t yet know how well AP503 or other activators of GPR133 will work in humans.
- The long-term safety is unclear. GPCRs are involved in many different processes across the body, so unwanted side effects are possible.
- The exact mechanism of how AP503 binds to GPR133 and how it interacts with mechanical loading still needs to be fully mapped.
- Human genetic differences may mean that not everyone responds the same way to a drug targeting GPR133.
Clinical trials will be needed to determine whether this discovery truly translates into a new class of osteoporosis medications.
A Quick Look at GPCRs
Since this research revolves around a GPCR, here’s a little more background:
- GPCRs are the largest family of cell surface receptors in humans, with over 800 different types.
- They detect everything from hormones to light to smells, making them essential for countless body functions.
- Roughly 30–40% of modern drugs work by activating or blocking GPCRs. These include medications for blood pressure, asthma, mental health, and more.
- Adhesion GPCRs, the subgroup GPR133 belongs to, are less studied but are increasingly linked to development, immunity, and tissue maintenance.
This means GPR133 research not only sheds light on bone health but also expands our knowledge of this underexplored receptor family.
Lifestyle and Mechanical Loading – Why Exercise Still Counts
One of the fascinating aspects of this discovery is how it ties into the role of mechanical loading. Exercise has long been known to improve bone strength. Weight-bearing activities like walking, jogging, and resistance training stimulate bone remodeling and increase density.
Now we understand part of the mechanism: receptors like GPR133 are turned on by strain and pressure in the bone. This means:
- Exercise remains a frontline defense against bone loss.
- Future drugs like AP503 might enhance the natural benefits of exercise.
- For people unable to exercise (due to illness, injury, or age), activating mechanosensitive pathways could act as a substitute.
Looking Ahead
The Leipzig University team is already planning follow-up projects to study GPR133 more closely and test AP503 in additional disease models. If the findings hold up in humans, this could mark the beginning of a new era in osteoporosis treatment.
It’s also possible that pharmaceutical companies will take this receptor as a starting point and design even more potent and specific drugs. Since the basic biology is now clear, drug development pipelines can begin exploring safety and dosing.
Conclusion
The discovery of GPR133 as a bone-strengthening receptor and the development of AP503 as its activator represent a major step forward in osteoporosis research. By promoting bone-building cells while inhibiting bone-destroying ones, and by working in sync with natural mechanical signals, this pathway offers a fresh and promising direction for medicine.
While there’s still a long road from mice to human treatments, the findings highlight the power of exploring underappreciated biological systems like adhesion GPCRs. For millions living with weak bones today, this research sparks new hope for better, safer therapies in the future.
Reference:
Lehmann J, Lin H, Zhang Z, Wiermann M, Ricken A, Brinkmann F, Brendler J, Ullmann C, Bayer L, Berndt S, Penk A, Winkler N, Hirsch FW, Fuhs T, Käs J, Xiao P, Schöneberg T, Rauner M, Sun JP, Liebscher I. The mechanosensitive adhesion G protein-coupled receptor 133 (GPR133/ADGRD1) enhances bone formation. Signal Transduction and Targeted Therapy. Published June 30, 2025. https://doi.org/10.1038/s41392-025-02291-y