Caltech Scientists Overturn Long-Held Model of Mitochondrial Protein Import
For decades, biology textbooks have stated that mitochondrial proteins—most of which are produced outside the organelle—are imported into mitochondria only after they are fully built in the cytosol.
Now, researchers at Caltech have shown that this long-standing model is incomplete. In fact, as much as 20 percent of mitochondrial proteins begin entering the organelle while they are still being made. This discovery provides a major revision of how we understand mitochondrial protein trafficking, with potential implications for both fundamental cell biology and future medical applications.
The Traditional View of Mitochondrial Protein Import
Mitochondria are often called the powerhouses of the cell because they generate ATP, the universal energy currency. These organelles have an evolutionary history as symbiotic bacteria that integrated into early eukaryotic cells more than a billion years ago. Over time, most of the mitochondrial genome was transferred to the nucleus. Today, mitochondria retain only a small number of genes, relying on the nucleus to encode the majority of their proteins.
Those nuclear-encoded proteins are built by ribosomes in the cytosol. The classic model states that once translation finishes, the completed protein—marked by a mitochondrial targeting sequence at its N-terminus—is recognized and then transported into the organelle through specialized pores in the mitochondrial membranes. For years, this post-translational import model was accepted as nearly universal.
Meanwhile, other organelles like the endoplasmic reticulum (ER) were known to use cotranslational import, where proteins enter the organelle while they are still attached to the ribosome and elongating. Mitochondria, however, were thought to be different. Cotranslational targeting to mitochondria was considered rare, if it happened at all.
The New Findings From Caltech
Shu-ou Shan, the Altair Professor of Chemistry at Caltech, along with her colleagues and former graduate student Zikun Zhu, decided to test this assumption with modern methods. They used selective ribosome profiling (SeRP), a technique that tracks ribosomes engaged with specific cellular complexes. By focusing on ribosomes associated with the TOM complex (the main protein gate of the outer mitochondrial membrane), they could determine whether mitochondrial proteins were being imported while still on the ribosome.
The results were surprising. About one-fifth of mitochondrial proteins in human cells showed evidence of cotranslational import. In other words, these proteins start entering the mitochondria during synthesis, not after. This discovery directly challenges the long-accepted textbook model.
Which Proteins Are Cotranslationally Imported?
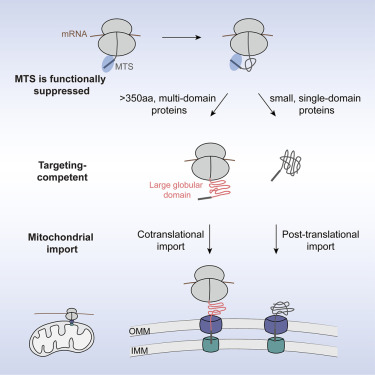
The researchers found that cotranslational import is not random. It is strongly biased toward large, topologically complex proteins that are particularly difficult to fold correctly once fully made. Many of these proteins contain residues that are distant from one another in the amino acid sequence but must align in precise three-dimensional arrangements. Such proteins are prone to misfolding or forming irreversible structures if they remain too long in the cytosol.
For these complex proteins, cotranslational import provides a way to avoid problems. By moving into mitochondria while still being synthesized, they bypass the risk of clogging or misfolding outside the organelle. This prevents blocked channels and ensures that the import machinery functions smoothly.
The Dual-Signal System of Import
The team also discovered that mitochondrial proteins carry two distinct signals that determine the timing of their import.
- Mitochondrial Targeting Sequence (MTS): Almost all nuclear-encoded mitochondrial proteins have this short amino acid sequence at their beginning. It acts as the protein’s “address label,” ensuring the protein is directed to mitochondria. However, this signal alone does not determine whether import occurs cotranslationally or post-translationally.
- First Large Foldable Domain: The critical additional factor is the emergence of a large protein domain during translation. When the ribosome synthesizes this first major structural unit, it acts like a trigger. At that point, the protein begins its journey into the mitochondrion. Without this domain, the targeting sequence alone is insufficient to start early import.
To test this, the researchers performed experiments transplanting these large domains onto other proteins that are normally imported only after translation. Remarkably, this rerouted the proteins to be imported during translation, confirming that these domains act as transferable signals.
Why Cotranslational Import Matters
The implications of this system are practical. If large, complex proteins were allowed to fully fold in the cytosol before entering mitochondria, they would risk forming stable, tangled structures that cannot pass through the narrow import channels. Cotranslational import prevents this by threading the protein through the pore before problematic folding occurs.
This process ensures efficiency and protects the cell from dangerous blockages. It also demonstrates that mitochondrial import is not a single uniform pathway but a multilayered and flexible system adapted to the needs of different proteins.
Differences From Other Organelles
One striking outcome of the study is that mitochondrial cotranslational targeting is fundamentally different from what happens in other organelles. For example, the ER preferentially imports membrane proteins during translation. In mitochondria, however, cotranslational import is biased toward soluble, multi-domain proteins that fold slowly and with difficulty. This highlights the evolutionary diversity of targeting pathways across organelles.
Experimental Approaches and Evidence
The study relied on several experimental strategies:
- Selective Ribosome Profiling (SeRP): Allowed the researchers to see exactly when mitochondrial proteins begin interacting with the TOM complex.
- Domain Swapping Experiments: By transplanting foldable domains, they demonstrated that the domain itself serves as a transferable signal for cotranslational import.
- Efficiency Comparisons: They compared proteins imported cotranslationally versus post-translationally and found that cotranslational targeting improved the efficiency of importing large, complex proteins.
Their datasets, including ribosome footprinting and TOM interactome profiles, are publicly available under accession number GSE255657 for further analysis by other researchers.
Broader Implications
This work fundamentally reshapes how scientists think about mitochondrial protein biogenesis. It shows that the cell uses a dual-check system—a targeting sequence plus a structural domain—to decide not only where a protein goes, but also when it goes there.
Beyond basic cell biology, this insight opens new research directions. If scientists can manipulate the timing of protein import, it could provide new tools for addressing mitochondrial diseases, many of which involve protein misfolding or trafficking defects. Synthetic biology and protein engineering could also benefit, with researchers designing proteins that are more efficiently targeted to mitochondria for specialized functions.
Additional Background: Why Protein Folding Is So Tricky
To fully appreciate the importance of cotranslational import, it helps to understand why protein folding can be a challenge:
- Proteins are chains of amino acids, but to function, they must fold into precise three-dimensional structures.
- Simple proteins can fold quickly because their neighboring amino acids interact easily.
- Large, complex proteins often require distant amino acids to align, which takes longer and carries more risk of misfolding.
- Misfolded proteins can aggregate into clumps, sometimes leading to disease. Cells deploy chaperones to assist folding, but the problem remains significant.
By importing proteins before they finish folding, mitochondria reduce the chances of misfolding outside the organelle.
Mitochondria: More Than Just Powerhouses
While this discovery focuses on import pathways, it also reminds us that mitochondria are not only energy producers. They play critical roles in:
- Metabolism of fats, amino acids, and nucleotides.
- Apoptosis, the programmed cell death pathway.
- Calcium storage and signaling, important for many cellular processes.
- Reactive oxygen species regulation, with implications for aging and disease.
Because mitochondria are so central to cell health, small improvements in how proteins are imported and folded can have large consequences for overall cellular function.
Looking Forward
The Caltech team’s work provides a blueprint for future studies. Scientists are now poised to investigate:
- How widespread cotranslational import is across different species.
- Whether defects in this pathway contribute to human diseases.
- How cells regulate the balance between cotranslational and post-translational import under stress conditions.
- Whether cotranslational import could be artificially engineered to improve mitochondrial therapies.
Funding and Collaborations
This research was supported by several institutions:
- National Institutes of Health (NIH) grant R35 GM136321 to Shu-ou Shan.
- Howard Hughes Medical Institute (HHMI) through a Freeman Hrabowski Scholar grant to Rebecca Voorhees.
- European Research Council (ERC) under Horizon 2020 grant no. 819318.
- Human Frontiers Science Program Organization, ref. RGP0016/2022.
- Israel Science Foundation, grant no. 1452/18.
These collaborations highlight the global interest in mitochondrial biology and its implications for health.
Conclusion
The discovery that cotranslational mitochondrial protein import is widespread overturns a decades-old assumption in cell biology. It shows that mitochondria, far from being passive recipients of fully built proteins, actively import a significant fraction of proteins during their synthesis. The dual-signal system—targeting sequence plus structural domain—provides an elegant solution to the problem of importing large, complex proteins efficiently.
By deepening our understanding of how mitochondria maintain their proteome, this study not only rewrites a fundamental chapter in cell biology but also opens potential new avenues for tackling diseases tied to mitochondrial dysfunction.
Reference: Principles of cotranslational mitochondrial protein import, Zhu et al., Cell, 11 August 2025