Scientists Reveal Carbon as the Key Ingredient Behind Earth’s Inner Core Formation
For decades, geoscientists have puzzled over how Earth’s inner core—a dense, iron-rich sphere at the planet’s center—was able to form in the first place. New research published in Nature Communications has finally shed light on this mystery, pointing to an unexpected hero: carbon.
This discovery not only helps explain how the solid inner core crystallized but also forces us to rethink what we know about the planet’s deep interior.
The Challenge of Explaining the Inner Core
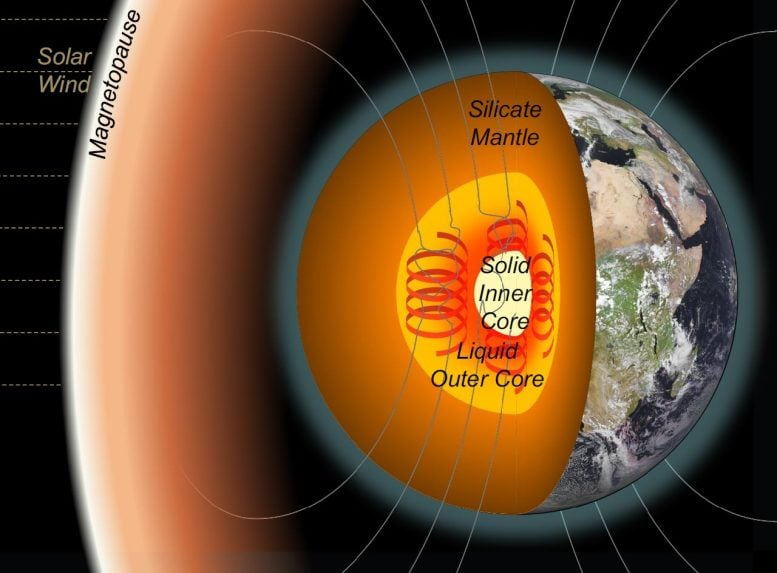
Earth’s inner core sits about 5,100 kilometers beneath the surface, surrounded by the molten outer core. It plays a critical role in powering Earth’s magnetic field, which protects us from solar wind and harmful space radiation.
But here’s the catch: forming a solid inner core isn’t as simple as iron cooling and hardening. For iron to crystallize under extreme pressure, it needs to undergo supercooling, meaning the liquid has to cool well below its normal freezing point before crystals begin to form.
Early models estimated that a pure iron core would need to supercool by around 800 to 1000 °C before it could begin to freeze. That doesn’t add up, because if the Earth’s core had cooled that much, the inner core today would be far larger than observed, and our planet’s magnetic field would likely have collapsed long ago. Clearly, something else had to be at work.
The Role of Light Elements
For years, scientists suspected that “light elements”—chemical elements lighter than iron—were mixed into the core and might influence crystallization. The main candidates included silicon, sulfur, oxygen, and carbon. These elements were thought to dissolve into the molten core during Earth’s early history.
Until now, though, it wasn’t clear which of these elements, if any, played the decisive role in triggering crystallization at the right time and under realistic cooling conditions.
Simulating the Deep Earth
Because we can’t directly sample the inner core, the research team—made up of scientists from the University of Oxford, University of Leeds, and University College London—turned to high-powered atomic-scale computer simulations.
They modeled a virtual system containing around 100,000 atoms, recreating the extreme pressures and temperatures found at the center of the planet. By tracking how often tiny crystal-like clusters of atoms formed within the molten environment, they were able to test how different elements influenced the nucleation (the first steps of freezing).
What the Simulations Showed
The results were surprising.
- Silicon and sulfur slowed freezing down. These elements made it harder for crystals to form, meaning even more supercooling would be required for solidification to begin. If silicon and sulfur dominated the inner core’s composition, Earth would have had a much harder time forming a solid inner sphere.
- Carbon accelerated freezing. In contrast, carbon lowered the supercooling requirement, making it easier for crystals to nucleate and grow.
To test this further, the researchers ran specific scenarios:
- With 2.4% carbon by weight, the inner core would need about 420 °C of supercooling. That’s still too much compared to what models suggest actually happened.
- But with 3.8% carbon by weight, the required supercooling dropped to just 266 °C. This value aligns with Earth’s actual thermal history and the observed size of the inner core.
The takeaway? Earth’s inner core likely contains more carbon than previously assumed, and this carbon was essential for its very existence.
Freezing Without “Seeds”
Another fascinating finding was that the core could freeze without nucleation seeds. Normally, in processes like water turning into ice, tiny impurities or particles act as seeds for crystals to form around. But in Earth’s core, any possible seed materials would have either melted or dissolved at such extreme conditions.
The simulations showed that with the right chemistry—specifically, the presence of carbon—the inner core could solidify naturally without any external help.
Why This Matters
This discovery has several major implications:
- Core composition: If the inner core contains around 3.8% carbon, then carbon is far more abundant in Earth’s deep interior than many earlier estimates suggested.
- Magnetic field stability: The crystallization of the inner core helps power the geodynamo that generates Earth’s magnetic field. Without carbon, the core might not have solidified, and the magnetic shield that protects life could be much weaker or nonexistent.
- Constraining Earth’s history: The timing of when the inner core first began to freeze has been debated for decades. Some estimates place it over 2 billion years ago, while others suggest less than 500 million years. Knowing that carbon played a key role narrows the range of possible scenarios for how the core cooled and evolved.
- Planetary comparisons: Understanding how Earth’s inner core crystallized could also help scientists compare our planet with other rocky bodies, like Mars or Mercury, and assess why their cores evolved differently.
Adding Context: What Exactly Is Supercooling?
Supercooling is when a liquid is cooled below its normal freezing point but doesn’t immediately solidify. This happens because forming the very first stable crystal requires an energy “push,” and without impurities or special conditions, the liquid can stay in a metastable state for a long time.
On Earth’s surface, supercooling is easy to demonstrate with water. Pure, clean water cooled carefully can stay liquid below 0 °C until something disturbs it—like tapping the container—causing it to suddenly freeze.
Inside Earth, however, supercooling is on a whole different scale. The required degree of cooling depends strongly on the chemical composition of the molten iron. That’s why identifying carbon as a key accelerator of crystallization is such a big breakthrough.
The Mystery of the Inner Core’s Age
One of the biggest open questions is still when the inner core began forming.
If crystallization started billions of years ago, the inner core would be quite large today, and the magnetic field history would look different. On the other hand, if it only began freezing in the last half a billion years, that would suggest Earth’s magnetic field has undergone dramatic changes more recently.
The new findings don’t pinpoint an exact age, but by showing that carbon enables crystallization with modest supercooling, they give scientists new constraints to refine thermal evolution models.
Carbon in Earth’s Story
Carbon is essential for life on the surface, but its role deep inside the planet has often been overlooked. This research shows that carbon also has a planetary-scale function—without it, Earth’s magnetic shield and habitability might never have developed.
Interestingly, Earth’s core is not thought to be made of iron alone. Seismic data and density models indicate that one or more additional light elements must be present. Carbon is now a prime candidate, but oxygen, silicon, and possibly hydrogen likely also contribute to the overall mix.
Broader Implications for Earth Science
This discovery fits into a larger scientific effort to answer fundamental questions:
- What exactly is inside Earth’s core? We can’t directly access it, but seismic waves, high-pressure experiments, and simulations provide clues.
- How do cores evolve in other planets? Mars, for example, no longer has a global magnetic field, possibly because its core cooled differently. Comparing planetary cores could reveal why Earth remains geologically active and habitable.
- How does deep chemistry connect to surface life? The link between carbon in the core and the persistence of a magnetic field shows just how interconnected Earth’s systems are—from the deepest interior to the outer atmosphere.
A Step Toward Solving the Puzzle
While this research marks a major step, it doesn’t close the book. The authors emphasize that carbon is unlikely to be the only element involved. A complete picture of Earth’s core composition will require understanding how multiple light elements interact with iron under extreme conditions.
Still, the central message is clear: without carbon, Earth’s solid inner core may never have formed. That means carbon isn’t just a building block for life—it may also have been essential in shaping the entire evolution of our planet.
Reference
Research paper: Constraining Earth’s core composition from inner core nucleation (Nature Communications, September 4, 2025).