Scientists at Johns Hopkins Discover a Way to “Supercharge” the Immune System to Stop Cancer from Coming Back
Researchers at Johns Hopkins All Children’s Hospital have found a new way to help the immune system recognize and destroy cancer cells—and possibly prevent cancer from returning. The team discovered that activating two specific immune pathways, called STING and LTβR, can convert so-called “immune-cold” tumors (the ones that usually hide from the immune system) into “immune-hot” tumors that immune cells can attack.
This discovery, published in Nature Immunology, provides a promising new approach to making cancers more responsive to treatments like immunotherapy and chemotherapy. It’s an important step forward in the long-running challenge of getting the immune system to do what it does best—fight off invaders, even when those invaders are cancer cells.
What the Scientists Found
Cancerous tumors often manage to stay under the radar of the immune system. These “immune-cold” tumors don’t give off the usual danger signals that trigger immune attacks, meaning that T cells and B cells—the white blood cells responsible for killing infected or abnormal cells—don’t rush in to destroy them. As a result, these tumors resist many treatments and are more likely to come back after therapy.
The Johns Hopkins team wanted to find a way to “hot-wire” the immune microenvironment inside these tumors, turning them from silent and unrecognizable masses into immune hotspots. To do that, they worked with mouse models of breast, pancreatic, and muscle cancers, simulating human tumor behavior as closely as possible.
By stimulating the STING (Stimulator of Interferon Genes) pathway and the lymphotoxin-β receptor (LTβR) pathway at the same time, the scientists created a reaction that dramatically changed how the immune system behaved inside the tumors. Dual activation of these two pathways led to the recruitment of T cells and B cells, the formation of immune “hubs” called tertiary lymphoid structures (TLSs), and the reshaping of tumor blood vessels into specialized immune gateways.
These specialized blood vessels, known as high endothelial venules (HEVs), acted like dedicated entry points for immune cells. They allowed large numbers of lymphocytes to enter the tumor tissue, helping to build local immune centers that functioned like miniature lymph nodes.
Building an Immune Infrastructure Inside Tumors
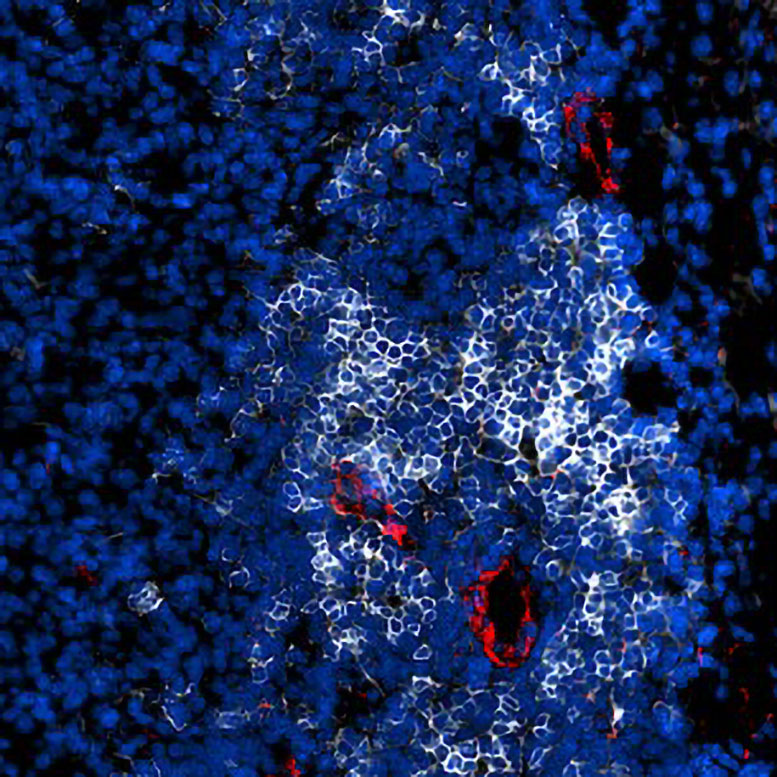
One of the most fascinating parts of the study was the discovery of how TLSs form and why they are so important. TLSs are clusters of lymphocytes that develop in areas of chronic inflammation, including certain tumors. In cancers that already have TLSs, patients tend to respond better to treatment and have longer survival rates.
To understand how TLSs form, the researchers first “reverse-engineered” tumors that already had TLSs to identify what triggered them. Then, they applied these same triggers to TLS-free tumors in mice. When they administered two immune-stimulating agents—one that activated STING and another that activated LTβR—they observed rapid changes.
Within a short time, the dual activation caused a surge of killer T cells (CD8⁺ T cells) into the tumors, leading to strong tumor growth inhibition. Meanwhile, B cells gathered and began forming dense TLS clusters surrounded by high endothelial venules. This process essentially built an immune ecosystem inside the tumor itself.
Inside these TLSs, B cells went through a transformation. They activated germinal center reactions, where B cells learn to make stronger and more specific antibodies. They then matured into plasma cells that secreted tumor-specific antibodies (IgG). Even after treatment, some of these plasma cells remained in the bone marrow, providing long-term immune memory—a kind of built-in vaccine effect that could help prevent cancer from returning.
The therapy also increased helper T cells (CD4⁺) and memory T cells (CD8⁺), strengthening both the antibody-mediated (humoral) and cell-mediated immune responses.
Why This Discovery Matters
This finding is significant because immune-cold tumors are one of the biggest obstacles in modern cancer treatment. These tumors don’t attract immune cells naturally, meaning that even powerful drugs like checkpoint inhibitors (which “release the brakes” on the immune system) often fail to work.
By converting these cold tumors into immune-hot ones, this approach could make previously unresponsive cancers vulnerable to immunotherapy. That’s a huge deal, especially for aggressive cancers like pancreatic and triple-negative breast cancer, which tend to resist standard treatments.
The study also provides insight into how the immune system can be restructured to fight cancer better. Instead of relying only on drugs that target the cancer cells directly, this approach builds immune infrastructure inside the tumor, allowing the patient’s own immune system to keep the cancer under control long-term.
The Role of STING and LTβR in Immunity
The STING pathway plays a central role in the body’s innate immune response. It’s part of the system that detects abnormal DNA in cells—like DNA from viruses or damaged cells—and triggers the release of interferons, powerful molecules that kickstart inflammation and immune activation. STING has been a hot topic in cancer research for years, but previous attempts to use STING agonists (drugs that activate the pathway) have often failed to show lasting benefits on their own.
The LTβR (lymphotoxin-β receptor), on the other hand, is crucial for the formation of lymph nodes and other lymphoid structures. It regulates how immune cells organize themselves and helps form high endothelial venules, which are the gateways for immune cell traffic.
What’s new in this study is the idea that activating both pathways simultaneously creates a synergy—STING brings the inflammation, while LTβR provides the organization. Together, they build a functional immune network right where it’s needed most: inside the tumor.
Experimental Details and Findings
The researchers conducted a series of experiments in mouse tumor models, using specific STING and LTβR agonists. When both were used together, the results were striking:
- Tumor growth slowed dramatically compared to single-agent treatments.
- Mice developed numerous TLSs and HEVs within their tumors.
- Depleting T cells or removing B cells prevented TLS formation, showing that both cell types are essential.
- Treated mice developed tumor-specific antibodies, which persisted in their systems long after treatment.
- In some models, mice treated with the dual therapy were protected from tumor recurrence even after re-exposure to cancer cells.
In other words, the immune system didn’t just fight the cancer—it remembered it.
A Look Toward the Future
While these results are extremely promising, they’re still preclinical—meaning all the experiments were done in animals. Translating this approach into humans will take time and careful testing. The main challenges ahead include:
- Safety: STING agonists can cause excessive inflammation if not carefully controlled.
- Delivery: Finding ways to deliver both agents directly to tumors without affecting healthy tissues.
- Variability: Human tumors are much more complex than those in mice, so results may vary widely between cancer types.
- Combination therapy: Determining how best to use this approach alongside existing treatments like checkpoint inhibitors or chemotherapy.
Despite these challenges, the findings open the door to a new class of combination immunotherapies designed not just to attack cancer but to re-engineer the immune system’s relationship with it.
Understanding “Hot” and “Cold” Tumors
To better appreciate this discovery, it helps to understand what scientists mean when they talk about immune-hot and immune-cold tumors.
- Immune-hot tumors are already inflamed and filled with immune cells. They often respond well to immunotherapy because the immune system can “see” the cancer. Examples include melanoma and certain lung cancers.
- Immune-cold tumors, by contrast, have very few immune cells and don’t send out distress signals. They often resist treatment and grow silently. Pancreatic cancer and some breast cancers fall into this category.
The goal of many modern immunotherapies is to turn cold tumors hot—to make them visible and vulnerable to attack. The Johns Hopkins study provides one of the clearest demonstrations so far of how that might be achieved.
The Promise of Tertiary Lymphoid Structures (TLSs)
TLSs have been observed in various cancers, autoimmune diseases, and chronic infections. Their presence inside tumors has been strongly linked to better survival outcomes.
What makes TLSs special is that they act as local command centers for the immune system. Inside these structures, B cells and T cells can communicate, train, and adapt their responses to the specific threats present in the surrounding tissue.
In a tumor, TLSs essentially allow the immune system to set up shop inside enemy territory. They’re not just clusters of cells—they’re functional mini-organs that help produce antibodies, memory cells, and targeted immune responses right at the site of disease.
The ability to therapeutically induce TLSs in immune-cold tumors is a major leap forward in immuno-oncology, and that’s exactly what this study demonstrates.
Support and Next Steps
The research received support from multiple institutions, including the National Cancer Institute/NIH, the Department of Defense Congressionally Directed Cancer Research Program, and the Florida Department of Health Bankhead Coley Cancer Research Program.
The Johns Hopkins team is now working to understand the finer details of how TLS therapy works at the molecular level and preparing for early clinical trials in both adult and pediatric cancer patients.
If this approach proves successful in humans, it could lead to a new generation of immune-based cancer treatments—ones that don’t just kill cancer cells, but teach the body to keep fighting them forever.