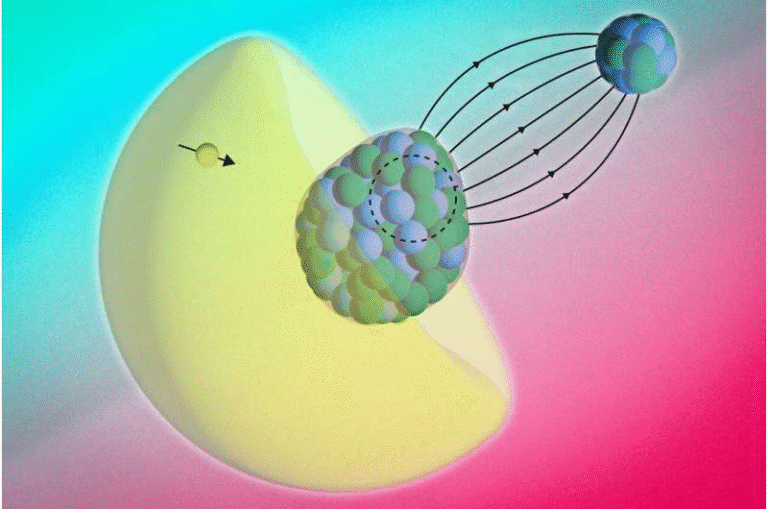Graphene’s Hidden Trick: It Only Partially Screens van der Waals Forces

A team of researchers from Peking University, Nanjing University of Aeronautics and Astronautics, and Tsinghua University has uncovered a fascinating property of graphene—it doesn’t completely block or fully transmit van der Waals (vdW) interactions.
Instead, it partially screens them, and this screening depends on how many graphene layers are present. Their study, published in Physical Review Letters (October 2025), shows that graphene layers screen between 15% and 50% of vdW forces, offering a nuanced understanding of how two-dimensional (2D) materials interact at the atomic scale.
What the Study Was About
Two-dimensional materials like graphene—just one atom thick—have revolutionized material science because they exhibit properties that differ sharply from their bulk counterparts. Researchers have long been interested in how these ultrathin materials interact with other surfaces through van der Waals forces, which are weak electrical attractions that arise between atoms or molecules due to fluctuating charges.
Understanding these interactions is more than a curiosity—it directly impacts how nano-devices, coatings, and electronic components behave. The key question has been: Does graphene let through the surface energy of the material beneath it, or does it block it?
This concept is often called “wetting transparency”—the idea that if you place a graphene sheet over another material, the properties of the underlying surface (like how water droplets spread on it) might still appear “visible” through graphene. Over the years, experiments have produced conflicting results: some suggested full transparency, others partial, and some even opacity. The new study sought to settle this long-standing debate with direct, quantitative data.
How the Researchers Tested Graphene’s Transparency
To answer the question, the researchers designed an experiment using colloidal atomic force microscopy (AFM)—a highly sensitive technique capable of measuring minute forces between surfaces.
Here’s what they did:
- They attached a micrometer-sized silica sphere to the tip of an AFM cantilever, creating a probe with well-known stiffness and geometry.
- They brought this probe close to two types of samples:
- Graphene supported on a silica (SiO₂) substrate, where the graphene lies flat on the surface.
- Graphene suspended across tiny circular cavities, so there’s no direct contact between graphene and the underlying substrate.
The environment was carefully controlled at ultra-low humidity (below 10%) to prevent water molecules from interfering with the delicate force measurements.
They performed two types of measurements:
- Pull-off tests, where they measured the force required to detach the probe from the surface.
- Pull-in tests, where they observed how the attractive force increased as the probe approached the surface.
By comparing data from the supported and suspended graphene, they could isolate how much of the underlying substrate’s vdW force passed through the graphene sheet.
What They Found
The results were consistent and revealing: graphene only partially screens vdW forces, and the degree of screening depends on both thickness and distance between the interacting surfaces.
- For single-layer graphene, when the probe was about 5 nanometers away, around 85% of the substrate’s vdW force still “leaked” through.
- As the number of layers increased, the screening effect became stronger. For up to five layers, the graphene screened between 15% and 50% of the substrate’s forces.
The takeaway: graphene isn’t completely transparent to van der Waals forces—it’s semi-transparent.
Why This Discovery Matters
This discovery goes beyond settling a scientific debate. It provides a quantitative foundation for understanding and engineering nanoscale interactions. Here’s why it’s important:
- Designing Better Coatings
The ability of graphene to partially transmit vdW forces means that it can be used to fine-tune adhesion and friction in advanced coatings. Engineers can adjust how sticky or slippery a surface is by controlling the number of graphene layers. - Improving Nano-Devices
At the nanoscale, vdW forces can cause parts to stick together (a problem known as “stiction”). Knowing exactly how these forces behave through graphene can help in designing more reliable nano-electromechanical systems (NEMS). - Advancing Heterostructure Engineering
In modern electronics, different 2D materials are stacked to create heterostructures. The screening effect determines how much one layer influences the other, affecting overall performance. This study gives researchers a tool to predict and control that influence. - Understanding Surface Energy at the Atomic Scale
The study provides clarity on the relationship between surface energy, adhesion, and vdW interactions. It’s a step toward understanding how single-atom coatings can modify the apparent properties of a material without chemically altering it.
The Broader Context: van der Waals Forces Explained
To fully appreciate this finding, it helps to understand what van der Waals forces are.
These are weak intermolecular forces that arise from temporary fluctuations in electron density around atoms. Even neutral atoms or molecules can experience a tiny, momentary dipole (positive and negative sides), which induces a similar dipole in neighboring atoms. The resulting attraction is what we call a van der Waals interaction.
Though weak individually, these forces become significant when billions of atoms are involved—like when two surfaces come close together. They’re crucial in determining how materials stick, adhere, or self-assemble.
In layered materials like graphene, these forces also hold the layers together. When you peel off a layer of graphene from graphite, you’re essentially overcoming vdW forces between sheets.
Understanding how graphene modifies these forces gives scientists a new handle on manipulating adhesion, friction, and surface energy at the smallest scales.
Graphene’s Special Role in Modern Science
Graphene is often called a “wonder material,” and for good reason. Composed of a single layer of carbon atoms arranged in a hexagonal lattice, it boasts:
- Exceptional strength (200 times stronger than steel)
- Outstanding electrical conductivity
- High flexibility
- Optical transparency
- Chemical stability
These properties have made graphene a cornerstone for next-generation electronics, sensors, composites, and coatings. However, as research advances, scientists are discovering that graphene’s interactions with its environment are more complex than first thought.
The current study adds an important piece to that puzzle—showing that even a one-atom-thick layer has measurable, tunable effects on fundamental forces.
Bridging Theory and Experiment
The researchers compared their findings with predictions from Lifshitz theory, a well-established framework that describes vdW interactions between materials separated by thin films. The theory predicts partial attenuation—exactly what the experiments confirmed.
The team’s data also help explain why earlier studies on wetting transparency (where scientists looked at water droplets on graphene-coated surfaces) produced contradictory results. Those experiments involved liquids, which add complications like surface tension and capillary forces. The new approach—directly measuring solid-solid vdW forces—sidesteps these complexities, offering cleaner, more interpretable results.
Beyond Graphene: What About Other 2D Materials?
While this study focused on graphene, the same principles apply to other 2D materials such as:
- Hexagonal boron nitride (hBN)
- Molybdenum disulfide (MoS₂)
- Phosphorene
- Transition metal dichalcogenides (TMDs)
Each material has unique electronic and dielectric properties that affect how it screens vdW interactions. Future research could explore whether these materials are more or less “transparent” than graphene and how they can be layered together for tailored adhesion or friction at the nanoscale.
Looking Ahead: Potential Applications
The implications of this research are wide-ranging. Some potential areas of application include:
1. Nanoelectronics
Graphene coatings could be used to control the contact resistance between electrodes and semiconductors, improving energy efficiency and reliability.
2. Flexible Devices
As flexible electronics become more common, understanding and controlling adhesion at atomic interfaces will be essential. Partial vdW screening gives engineers a new design parameter.
3. Photonics and Sensors
Tuning surface energy through graphene coatings could improve the performance of optical and sensing devices, where light-matter interaction depends on surface properties.
4. Advanced Manufacturing
In processes like thin-film deposition, knowing how much of the substrate’s influence “leaks through” can help achieve smoother, more consistent coatings.
5. Space and Vacuum Technologies
Because the experiment was done in dry, low-humidity environments, the findings also have relevance for vacuum systems, where graphene coatings could be used to manage adhesion and contamination at micro and nano levels.
Limitations and Next Steps
Like any precise scientific study, this work has boundaries. The results apply specifically to graphene on silica and to conditions of low humidity. In real-world applications, contaminants, surface roughness, or ambient humidity could change the magnitude of vdW forces.
Nevertheless, the researchers plan to extend their work to engineer silica surfaces with atomic coatings that have customizable adhesion properties. This could lead to better integration of functional layers and electrical contacts in microelectronics.
The broader message is clear: even at the atomic scale, there’s room to engineer and control how materials “feel” each other’s presence.
Final Thoughts
This study brings new precision to a subtle question: how does a one-atom-thick sheet of graphene influence the invisible but omnipresent forces that make matter stick together? The answer—graphene partially screens van der Waals interactions depending on its thickness—opens up new directions for both fundamental physics and practical engineering.
In a world moving ever closer to atomic-level design, knowing how much of one surface’s “force field” reaches another isn’t just academic curiosity—it’s the foundation for the next generation of miniaturized, efficient, and adaptive materials.
Research Reference:
Transparency of Graphene to Solid–Solid van der Waals Interactions – Physical Review Letters (2025)