New Molecular Dam Design Boosts Nanocrystal Efficiency for Light-Driven Reactions
Researchers from the University of Colorado Boulder, University of California, Irvine, and Fort Lewis College have developed a clever new way to stop energy from leaking out of semiconductor nanocrystals—a long-standing problem that has limited their use in light-powered chemical reactions. This breakthrough, published in Chem (2025), introduces a “molecular dam” that holds back energy, significantly extending how long light-generated charges last inside nanocrystals.
This simple but powerful concept could make photocatalysis—the use of light to power chemical reactions—much more efficient, paving the way for greener ways to produce essential materials like plastics, fertilizers, and pharmaceuticals.
The Challenge of Energy Leaks in Nanocrystals
When light hits a semiconductor nanocrystal, it excites electrons, creating a negative electron and a positive “hole” (the absence of an electron). This process, known as charge separation, briefly stores energy that could be used to power chemical reactions. But there’s a catch: these electrons and holes tend to recombine extremely quickly, wasting the energy as heat before it can be used.
Scientists have spent years trying to find ways to keep the charges apart for longer, giving chemical reactions enough time to occur. In most cases, the charge-separated state lasts for only nanoseconds—a blink of an eye in the quantum world. For photocatalysis to be practical, researchers need to stretch that timescale by several orders of magnitude.
That’s where the molecular dam comes in.
How the “Molecular Dam” Works
The team focused on cadmium sulfide (CdS) nanocrystals, a well-studied material in photochemistry. Their goal was to design a molecule that could bind to the nanocrystal’s surface and capture one of the charges before it recombined with its opposite partner.
They chose a phenothiazine derivative—a molecule known for its ability to accept positive charges (holes). But instead of just letting it float around near the nanocrystal, they modified it to stick tightly to the crystal’s surface.
Here’s how they did it:
- The molecule was given a carboxylate group—a sticky chemical “anchor” that binds strongly to the nanocrystal surface.
- The rest of the molecule was designed to quickly grab the hole generated by light absorption.
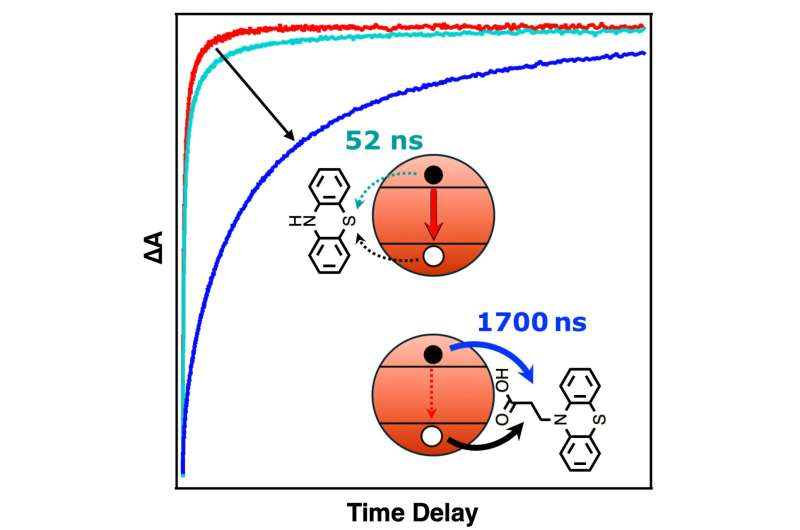
Once this anchored molecule is attached, the system behaves differently. When light hits the CdS nanocrystal, it creates an electron-hole pair as usual. But almost immediately, the phenothiazine molecule pulls the hole away and holds it at a safe distance from the electron. This physical and electronic separation prevents the two from recombining too quickly.
The result? The charges remain separated for microseconds instead of nanoseconds—thousands of times longer than before.
Record-Breaking Charge Separation Lifetimes
To test the system, the researchers compared two versions of the phenothiazine molecule:
- With the carboxylate anchor, which binds to the CdS nanocrystal.
- Without the anchor, which cannot attach firmly to the surface.
The difference was dramatic. The anchored version performed far better, proving that strong surface binding is essential for extending charge lifetimes.
In the best-performing configuration—CdS nanorods with the bound phenothiazine derivative—the researchers measured a charge-separated state lasting up to 24.2 microseconds. That’s an enormous leap in photochemical timescales.
Even more impressively, this led to roughly a 43× improvement in photocatalytic efficiency compared to systems without the anchored molecule. For photochemistry, where even small gains can mean a lot, that’s a huge step forward.
The Collaborative Effort Behind the Discovery
This project was truly collaborative. Each research group brought unique expertise to the table:
- Fort Lewis College: Kenny Miller’s group of undergraduate researchers synthesized the carboxylated phenothiazine derivatives, testing multiple variations.
- UC Irvine: Jenny Yang’s team, specialists in inorganic electrochemistry, performed electrochemical characterization of the molecules to understand how they transferred charges.
- CU Boulder: Gordana Dukovic’s group synthesized the CdS nanocrystals, attached the phenothiazine molecules, and used advanced laser spectroscopy to monitor how electrons and holes behaved after light absorption.
When the CU Boulder team first saw how effectively the molecular dam slowed charge recombination, they realized they had discovered something significant. The molecular system managed to slow recombination from nanoseconds to microseconds, something never before achieved with these materials.
Why This Matters for Photocatalysis
Photocatalysis has been a dream technology for decades. The idea is to use light, instead of heat or fossil fuels, to drive chemical reactions. If perfected, it could revolutionize industries that currently depend on energy-intensive, high-temperature reactors.
Many products we use daily—like plastics, fertilizers, fuels, and pharmaceuticals—require enormous energy to produce. Much of this energy comes from burning fossil fuels, releasing carbon dioxide and contributing to climate change. Photocatalysis offers a cleaner, more sustainable path forward: using sunlight directly to power the same reactions at room temperature.
But the bottleneck has always been efficiency. If light-generated charges recombine too fast, the energy is wasted. That’s why extending the charge lifetime from nanoseconds to microseconds is such a breakthrough—it finally gives enough time for useful chemical transformations to happen.
The Bigger Picture: Toward Light-Driven Chemical Manufacturing
This discovery doesn’t just help one specific type of reaction—it opens the door to improving a whole range of light-driven processes. The concept of attaching “charge-managing” molecules to nanocrystal surfaces could be adapted to many different materials and reaction types.
The researchers envision a future where we could manufacture essential chemicals using sunlight instead of fossil fuels. Imagine solar-powered chemical plants that synthesize polymers, fuels, or even pharmaceuticals under ambient conditions—no giant furnaces or high-pressure reactors required.
That vision is still in development, but this “molecular dam” represents a crucial piece of the puzzle. It shows how careful molecular design can control what happens at the nanoscale, leading to macroscopic improvements in efficiency.
The Science Behind It: What Are Nanocrystals?
To understand why this matters, it helps to know what nanocrystals are. These are tiny particles—often just a few nanometers across—made of semiconductor materials like cadmium sulfide (CdS), cadmium selenide (CdSe), or lead sulfide (PbS). For comparison, a human hair is about 100,000 nanometers wide.
Nanocrystals are special because of quantum confinement. When materials shrink to this size, their electronic properties change dramatically. They can absorb and emit light at different wavelengths depending on their size, making them useful in LEDs, solar cells, and quantum dots used in displays.
But in photocatalysis, the key property is their ability to absorb light and create charge carriers—the electron and hole pair that can drive reactions. The challenge, as this research highlights, is preventing those carriers from recombining too quickly.
The Role of Surface Chemistry
The surface of a nanocrystal is critical to its behavior. Because nanocrystals have such a high surface-to-volume ratio, what’s on their surface often matters as much as what’s inside.
In this study, the carboxylate anchor acts as a kind of “molecular glue” that allows precise control over surface interactions. By covalently binding the phenothiazine molecule to the CdS nanocrystal, the researchers created a robust and stable hybrid system where charge transfer can occur efficiently.
Surface chemistry like this is becoming a key tool in nanoscience. By customizing what molecules stick to the surface, scientists can fine-tune how nanomaterials behave—whether for catalysis, energy storage, or sensing.
Implications for Future Technologies
The design principles demonstrated here can guide the development of new photocatalysts and light-driven systems far beyond CdS. The concept of a molecule that both binds tightly and captures charge can be extended to more environmentally friendly materials that avoid toxic elements like cadmium.
It could also inform research in solar fuels, where scientists aim to split water into hydrogen and oxygen or convert carbon dioxide into fuels using sunlight. Extending charge lifetimes is a key requirement for making those processes efficient.
This molecular dam approach adds a new tool to the growing chemical toolkit for light-based energy conversion.
Funding and Acknowledgments
This research was supported by the U.S. Department of Energy’s Ensembles of Photosynthetic Nanoreactors (EPN) Energy Frontier Research Center under grant DE-SC0023431, with additional support from the Air Force Office of Scientific Research (AFOSR) under FA9550-22-1-0347. It highlights how interdisciplinary collaboration—across chemistry, physics, and materials science—can lead to breakthroughs in sustainable energy.
Conclusion
The molecular dam discovery marks a major leap forward in controlling charge dynamics inside nanocrystals. By extending charge separation lifetimes from nanoseconds to microseconds, the team has created a system that’s far more capable of using light efficiently to power chemical reactions.
This is not just a small technical achievement—it’s a foundational step toward light-driven chemical manufacturing, where we could one day make essential materials directly from sunlight. The approach shows that sometimes, solving a complex energy problem comes down to the right molecule in the right place—holding back energy just long enough to make a difference.
Research Paper:
Exceptionally long-lived charge-separated states in CdS nanocrystals with a covalently bound phenothiazine derivative (Chem, 2025)