Underwater Hydrothermal Vents May Have Sparked the First Steps Toward Life on Earth
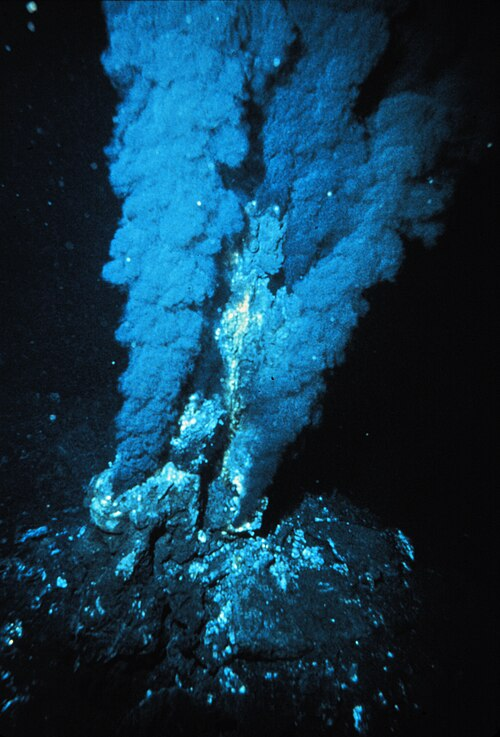
A fascinating new study published in the Journal of the American Chemical Society suggests that Earth’s earliest chemistry of life might have started deep under the ocean, at the mysterious alkaline hydrothermal vents. These vents, found on the seafloor, are natural openings where hot, mineral-rich fluids gush out, creating strong contrasts in temperature, acidity, and electric charge compared to the surrounding seawater. Researchers have now recreated these extreme conditions in the lab — and the results could reshape how we think life began.
Recreating the Chemistry of Early Earth
The team of scientists from Brazil, Japan, the United Kingdom, and the United States designed experiments to simulate how the first molecular precursors of life could have formed roughly four billion years ago, during the Hadean eon, the oldest era in Earth’s history.
Using specially built bench-scale reactors, they mimicked the interaction between hot, alkaline fluids (around 70°C, with a pH of 9–12) and cold, slightly acidic ocean water (around 5°C, with a pH near 5.5). This temperature and pH difference naturally creates an electrochemical gradient — a sort of primitive “battery” where electrons can flow from one side to the other.
Inside the reactor, they placed minerals commonly found in hydrothermal vents: iron–sulfur (Fe–S) and iron–nickel–sulfur (Fe–Ni–S) compounds. These minerals, surprisingly, resemble the metal centers of enzymes that power modern biological metabolism. In the experiment, the minerals acted as natural catalysts, helping convert carbon dioxide (CO₂) into simple carbon-based molecules without using any biological enzymes.
What Happened in the Experiment
When the scientists allowed the hot and cold fluids to interact through the mineral barrier, CO₂ was reduced to formic acid (HCOOH) — a simple organic acid — and then to acetic acid (CH₃COOH). These are not just random molecules; they are crucial intermediates in what is known as the Wood–Ljungdahl pathway, one of the oldest biochemical routes for carbon fixation still used today by some bacteria.
The team detected micromolar concentrations of these compounds on the “oceanic” side of the reactor, confirming that natural redox reactions (electron transfers) were taking place. The presence of nickel in the minerals enhanced the yield of formic acid, showing that nickel plays a catalytic role similar to its role in modern metabolic enzymes.
Perhaps most strikingly, the researchers found that tiny electrical currents — on the order of nanoamperes (10⁻⁹ amperes) — were sufficient to drive the reaction. That means even the faint natural currents flowing through minerals at the ocean floor billions of years ago could have powered early protometabolic reactions, long before life had evolved any enzymes or membranes.
Why This Matters: Protometabolism Without Life
This experiment is powerful because it bridges the gap between geochemistry and biochemistry. It demonstrates that life’s earliest chemistry didn’t necessarily require complex organic soups or enzymes. Instead, it could have begun as simple energy-driven reactions occurring at the interface between hydrothermal fluids and primitive seawater.
This concept is often called “protometabolism” — a form of pre-life chemical network that mimics metabolism but is purely mineral-driven. The natural voltage generated by hydrothermal vents could act like the membrane potential in modern cells, which cells use to generate energy in the form of ATP (adenosine triphosphate).
In other words, before life even existed, the planet itself might have been running a kind of primitive metabolism, powered by its own internal chemistry.
How the Natural “Battery” Works
At the heart of this discovery lies the idea of electrochemical gradients. When hot, alkaline vent fluids mix with cold, acidic seawater, they create zones with drastically different chemical potentials. Minerals formed at this boundary — especially iron and nickel sulfides — conduct electrons. The result is a natural voltage difference that can drive redox reactions, similar to how modern biological systems work.
For example, in living cells, mitochondria use voltage differences across membranes to fuel reactions that convert nutrients into energy. The same principle might have applied in ancient oceans — but instead of enzymes and membranes, minerals and gradients did the job.
This natural energy system could have sustained continuous CO₂ reduction and laid the groundwork for the emergence of life, one reaction at a time.
The Role of the Wood–Ljungdahl Pathway
The Wood–Ljungdahl pathway, named after biochemists Harland Wood and Lars Ljungdahl, is still used by certain bacteria today, especially acetogens and methanogens. It’s considered one of the most ancient carbon fixation mechanisms, capable of transforming CO₂ into acetyl-CoA, a molecule essential to metabolism.
The pathway relies on hydrogen as an electron donor, just like in these hydrothermal vent experiments. The study successfully reproduced the first two steps of this pathway — the reduction of CO₂ to formate, and then to acetate — without enzymes, relying solely on minerals and natural currents.
This supports the idea that the core chemistry of life could have existed long before cells or enzymes evolved. These early reactions might have eventually given rise to biochemical networks once organic molecules and membranes appeared.
Implications Beyond Earth
The implications of this research go beyond Earth’s history. Similar conditions — underwater hydrothermal activity, chemical gradients, and mineral catalysts — are believed to exist on Jupiter’s moon Europa and Saturn’s moon Enceladus, both of which have subsurface oceans.
If life on Earth began through such processes, these worlds could host similar prebiotic chemistry or even primitive life forms today. That’s why hydrothermal vents are among the prime targets for astrobiological exploration.
Modern Technological Applications
Beyond understanding life’s origins, this research also offers inspiration for modern technologies. The same type of mineral catalysts and natural gradients could be used in electrocatalysis — processes that use electricity to drive chemical reactions.
For example:
- Hydrogen production: Simulating vent-like reactions could make hydrogen fuel generation more efficient and sustainable.
- CO₂ reduction: The process could be adapted to develop systems that convert CO₂ into useful chemicals or fuels, helping combat climate change.
The study’s insights show how nature’s own energy systems might inspire cleaner and smarter engineering designs for the future.
The Broader Context: The Hadean World
To appreciate this discovery, it helps to imagine the Hadean Earth. Around 4.6 to 4.0 billion years ago, our planet was a violent, hot world constantly bombarded by asteroids. The oceans were acidic, the atmosphere lacked oxygen, and yet — beneath the surface — chemical energy was abundant.
At that time, hydrothermal vents would have created dynamic zones rich in minerals and hydrogen, constantly mixing with the cold ocean. These zones could have served as chemical nurseries, producing small organic molecules that later combined into more complex structures, setting the stage for the first living systems.
Challenges and Next Steps
While the results are impressive, this study represents one piece of a much larger puzzle. The reactions produced only small amounts of organic molecules (micromolar concentrations), and the researchers have not yet connected these steps to the creation of more complex compounds like amino acids or nucleotides.
Nevertheless, this experiment shows direct experimental evidence that Earth’s natural geochemistry could power the first steps toward life — something scientists have theorized for decades but struggled to demonstrate convincingly.
Future research may focus on expanding these reactions, testing longer carbon chains, or examining how minerals could have acted as templates for forming biological polymers.
Final Thoughts
The findings remind us that life’s origins might not be a cosmic miracle, but a natural consequence of Earth’s chemistry and physics. Hydrothermal vents — once seen merely as geological curiosities — could, in fact, be the cradles of life.
And if life began in the darkness of the ocean floor, powered by minerals and gradients, it might still be quietly emerging elsewhere in the universe, waiting for someone to notice.
Research Reference:
Carbon Reduction Powered by Natural Electrochemical Gradients under Submarine Hydrothermal Vent Conditions – Journal of the American Chemical Society (2025)





