New Imaging Technique Reveals Hidden Hydrogen Intermediates That Could Supercharge Clean Energy Research
A team of Cornell University researchers has developed a powerful new imaging technique that lets scientists actually see fleeting chemical intermediates during hydrogen electrocatalysis — reactions that are essential for clean hydrogen production and pollutant removal. The work, published in Nature Catalysis (October 2025), could change how scientists understand and design catalysts for a sustainable energy future.
The Challenge of Seeing the Invisible
When scientists talk about electrocatalysis, they’re referring to reactions that use electricity to drive chemical transformations — for example, splitting water into hydrogen and oxygen. But these reactions don’t happen in one smooth step. They go through transient intermediate states, such as metal-hydrogen species (M–H*), which form on a catalyst’s surface before transforming into the final product.
The problem? These intermediates are extremely short-lived and exist in tiny amounts. Traditional tools average the signal from millions of particles, which blurs the fine details. That means we’ve been missing a lot of what actually happens on the surface of the catalyst — especially the variations from one nanoparticle to another, or even from one site to another on the same particle.
That’s where the Cornell team, led by Peng Chen (Peter J.W. Debye Professor of Chemistry) and former postdoctoral researcher Wenjie Li, comes in. They wanted to see these elusive intermediates directly, at the level of individual molecules.
The Breakthrough: Single-Molecule Reaction Imaging
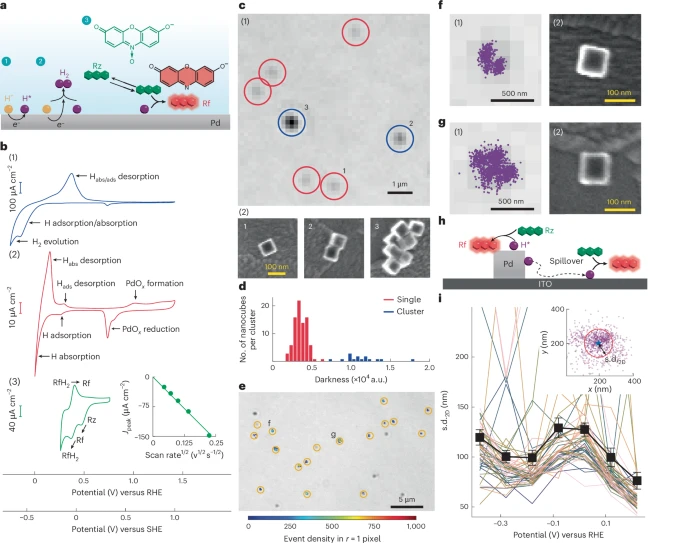
The researchers used a technique called single-molecule super-resolution reaction imaging, which allowed them to map and monitor chemical reactions on single catalyst particles with nanometer precision.
To do this, they focused on palladium-hydrogen (Pd–H*) intermediates — a classic model system for hydrogen-related reactions. They introduced a special probe molecule that reacts specifically with Pd–H* intermediates on the surface of tiny palladium nanocubes. When the probe reacts, it creates a fluorescent molecule — essentially a bright signal that can be captured by advanced microscopy.
This fluorescence isn’t just a pretty light show. Each bright spot represents a single chemical event, letting the team determine where and how fast these reactions occur. Because they can pinpoint each event’s location within a few nanometers, they can literally create a map of chemical activity across a single nanoparticle.
What the Researchers Found
The results were both fascinating and revealing.
- Each nanoparticle behaved differently. The researchers discovered that individual palladium nanocubes displayed diverse hydrogenation behaviors. In other words, not all catalyst particles perform the same, even if they are made of the same material and shape.
- Even within one nanoparticle, different sites showed unique reactivity. Some areas of a single particle were much more active than others. This intra-particle variation means that real catalysts are far more complex than previously thought.
- Hydrogen isn’t stationary — it moves. Once hydrogen atoms attach to the palladium surface, they don’t stay still. They can migrate across the surface or even spill over onto neighboring non-metallic areas. The researchers were able to visualize this hydrogen spillover directly for the first time, observing that it could extend hundreds of nanometers from the original metal surface.
- Existing analysis methods overestimate stability. Many scientists use so-called ensemble-averaged methods to study such intermediates in bulk. The Cornell team compared their precise imaging data with a Gaussian-broadening kinetic analysis, revealing that bulk methods tend to overestimate how stable these intermediates are. The reason? Averaging hides the fast, unstable reactions and overrepresents the slower, more stable ones.
- They identified distinct subpopulations of behavior. Instead of uniform performance, the palladium particles fell into three subgroups — each showing different patterns of intermediate stability, reaction speed, and overall behavior. This level of granularity had never been seen before in electrocatalysis research.
Why This Matters for Hydrogen Energy
Hydrogen is one of the most promising clean energy carriers of the future. Efficient hydrogen evolution reactions (HER) are critical for technologies like water electrolysis — a method to produce hydrogen using renewable electricity. Understanding how hydrogen interacts with catalysts is key to making this process faster, cheaper, and more reliable.
The new imaging technique helps scientists see the hidden steps that determine whether a catalyst performs well or not. For example, if hydrogen intermediates are too stable, they can block active sites and slow the reaction. If they’re too unstable, they disappear before contributing to hydrogen production. Striking the right balance requires knowing what’s happening on a site-by-site level — something this new method can finally reveal.
This could also help in environmental applications. Hydrogen-based electrocatalysis can break down pollutants like chlorinated compounds in water. By understanding where and how the hydrogen intermediates form and migrate, scientists could design better catalysts for water detoxification and green chemical manufacturing.
What Is Hydrogen Spillover?
Since “hydrogen spillover” plays a big role in this research, it’s worth unpacking. The term describes a process where hydrogen atoms move from a metal catalyst surface to the surrounding support material, like carbon or metal oxides.
This phenomenon has been known for decades, but until now, it had never been directly visualized during an active electrocatalytic reaction. The Cornell study showed that the hydrogen atoms can travel hundreds of nanometers, far beyond the metal surface, which expands how we think about catalyst design. Supports may play a more active role in these reactions than scientists realized.
Understanding spillover could lead to new hybrid catalyst architectures, where both the metal and the support contribute to the overall reactivity. That could open up more efficient pathways for hydrogen production, storage, and even fuel cell operation.
Broader Impact and Future Directions
The approach demonstrated by Li and Chen’s team is not limited to palladium or hydrogen. Because it’s based on fluorescence detection of reaction intermediates, it could be adapted to study many other electrocatalytic systems, such as:
- CO₂ reduction, where carbon dioxide is converted into fuels or chemicals.
- Nitrogen reduction, a pathway to make ammonia sustainably.
- Oxygen evolution and reduction, critical reactions in fuel cells and metal-air batteries.
By providing nanoscale insight into where reactions actually happen, this imaging technique could transform how researchers think about catalyst optimization. Rather than treating catalysts as uniform materials, scientists can now identify and enhance the most active regions, improving efficiency without relying solely on trial-and-error synthesis.
Future work could involve applying this technique to other metals (like platinum or nickel) or exploring how different supports — such as oxides or carbon materials — influence spillover distance and reaction kinetics. Another exciting possibility is watching how these intermediates behave under real operating conditions, like in working electrolyzers or reactors.
The Takeaway
This study is a major step forward in understanding how hydrogen behaves on catalyst surfaces. By capturing real-time images of electrocatalytic intermediates, the Cornell researchers have opened a window into a world that was previously invisible. Their discovery of heterogeneous reactivity, hydrogen mobility, and hidden variations between particles and sites could lead to more efficient, sustainable technologies for both energy production and environmental cleanup.
In short, by learning where hydrogen goes and how it behaves, we can build better catalysts — and move closer to a clean energy future powered by hydrogen.
Research Reference:
Single-molecule reaction mapping uncovers diverse behaviours of electrocatalytic surface Pd–H intermediates, Nature Catalysis (2025)