A Bacterium That Eats Battery Waste: The Future of Self-Sufficient Recycling
A group of researchers at Boston College has made a surprising discovery — a type of bacterium that can literally feed on battery waste. The study, published in ACS Sustainable Resource Management (2025), reveals how this microbe could help revolutionize the way we recycle used batteries, cutting down on energy costs and eliminating toxic chemicals in the process.
This is not some far-fetched idea. It’s a real experiment involving a real bacterium — Acidithiobacillus ferrooxidans — and it has shown that microbes might one day make battery recycling cleaner, cheaper, and self-sufficient.
The Core Discovery
The bacterium Acidithiobacillus ferrooxidans (Atf) thrives in acidic environments and is already known for its ability to oxidize iron and sulfur compounds. What’s new here is that researchers found it can use materials from spent batteries — specifically iron — as a nutrient source.
In the study, the team led by Professor Dunwei Wang (Chemistry) and Associate Professor Babak Momeni (Biology) explored whether Atf could grow and function using iron casing materials from used batteries instead of traditional nutrients.
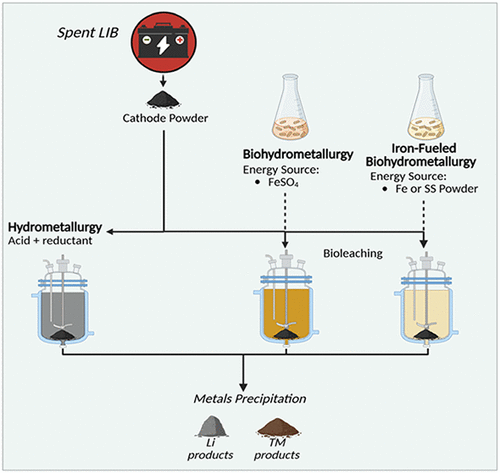
The results were promising. The bacterium not only survived but thrived, producing protons that effectively leached valuable materials from the battery cathodes. This leaching process — where acidic or biological agents dissolve metals — is a key step in battery recycling.
What makes this significant is that the bacterium powered itself using the battery waste it was recycling. This circular process, where the waste material fuels the recycling process, is what the researchers call “self-sufficient battery recycling.”
Testing Different Materials
Initially, the researchers tested pure iron, which is often found as a casing material in older batteries. But they didn’t stop there. They went further and tested stainless steel, a more common component in modern batteries.
Surprisingly, stainless steel worked even better than pure iron as a food source for the bacteria. This was unexpected because stainless steel is a complex alloy containing iron, chromium, and nickel — not exactly an easy meal for microbes. Yet, Atf managed to use it efficiently, showing even higher levels of leaching activity.
This discovery makes the process more relevant to real-world applications, since most modern batteries are housed in stainless steel casings.
Breaking Free from Sulfates
Traditional bioleaching (biological metal extraction) usually depends on sulfate compounds, which serve as energy sources for bacteria. However, sulfates are toxic, expensive to transport, and environmentally problematic.
The Boston College team demonstrated that Atf can function even in low-sulfate or sulfate-free conditions, maintaining strong leaching activity. This is a breakthrough finding because it removes one of the biggest logistical hurdles in scaling bio-based recycling systems — the need to supply and manage hazardous sulfate materials.
In short, the bacterium doesn’t need help. It can do its job with minimal additives and no toxic chemicals.
Why This Matters for the Future
Battery recycling is one of the most pressing technological and environmental challenges today. We live in a society increasingly powered by lithium-ion batteries — from smartphones and laptops to electric vehicles and renewable energy systems.
However, producing new batteries requires mining vast quantities of metals like lithium, cobalt, and nickel, which is energy-intensive and often harmful to the environment. At the same time, billions of used batteries are piling up in landfills, leaking toxic chemicals into the soil and water.
Traditional recycling methods — pyrometallurgical (high-temperature smelting) and hydrometallurgical (acid-based extraction) — are effective but require huge energy input, create toxic byproducts, and involve transporting hazardous materials.
The biological method demonstrated by Atf offers a low-energy, eco-friendly alternative. It has the potential to:
- Reduce dependence on chemical reagents and high temperatures.
- Minimize waste and emissions.
- Allow local recycling facilities to operate without complex infrastructure.
- Recover valuable metals that can be reused to build new batteries.
In essence, this could lead to self-sustaining recycling plants powered by bacteria rather than fossil fuels.
The Team Behind the Work
The project was a collaborative effort between scientists from different disciplines:
- Prof. Dunwei Wang, a physical chemist focusing on clean energy technologies, led the recycling experiments.
- Assoc. Prof. Babak Momeni, who specializes in microbial ecology and mathematical modeling, handled the cultivation and growth optimization of the bacteria.
- Other contributors include research associate Wei Li, graduate student Brooke Elander, and undergraduates Mengyun Jiang and Mikayla Fahrenbruch.
Their teamwork bridged microbiology and materials chemistry — a classic example of how interdisciplinary research can yield unexpected solutions.
What Comes Next
The researchers are now working on evolving the bacterium to improve its recycling efficiency. In simple terms, they are trying to make it “stronger” — better at breaking down waste, faster at processing materials, and more resilient in industrial settings.
They are also experimenting with building prototype batteries using the materials recovered by the bacteria. The goal is to see if these recycled components can match the performance of traditional, newly manufactured batteries.
If the recycled materials perform well, this will be a critical validation — showing that sustainable recycling can also deliver top-tier battery quality.
How Acidithiobacillus ferrooxidans Works
To understand why this bacterium is so special, it helps to know a little about how it works.
Acidithiobacillus ferrooxidans is known as a chemolithoautotrophic organism — meaning it gains energy by oxidizing inorganic substances (like iron and sulfur) rather than consuming organic food like sugars or fats.
It thrives in acidic environments (pH 1.5 to 3) and is already used in bioleaching for mining, especially for extracting copper and gold from ores. In those cases, it helps dissolve metals naturally through its metabolism.
What’s unique in this study is that the bacterium’s natural acid-producing metabolism is harnessed for battery recycling instead of mining. It’s essentially being redirected from ore to electronic waste, offering a biological alternative to chemical leaching.
This reuse of microbial power represents a new branch of green biotechnology — one that could one day make electronic waste recycling a much more sustainable process.
Broader Environmental and Economic Impact
If scaled successfully, this technique could have massive global implications. For instance:
- Developing countries that lack industrial-scale recycling plants could adopt microbial recycling systems locally.
- It could drastically cut the carbon footprint of battery production, reducing reliance on energy-hungry mining operations.
- Since the bacteria thrive in acidic environments and don’t rely on external sulfate sources, the process could be easily adapted for different battery chemistries and waste compositions.
There’s still a long way to go — optimizing efficiency, controlling contamination, and ensuring cost-effectiveness — but this approach adds a powerful new tool to the clean-tech arsenal.
A Glimpse into a Microbial Recycling Future
Imagine a future where recycling plants are alive — filled not with roaring furnaces but with bubbling bioreactors teeming with hardworking microbes. These tiny organisms could digest our electronic waste, recover valuable materials, and help close the loop on our ever-growing demand for batteries.
That’s the vision this study points toward: a self-sufficient, eco-friendly recycling system powered by biology rather than brute-force chemistry.
Research Reference:
Recycling Li-Ion Battery Cathode Materials in Iron-Fueled, Low-Sulfate Cultures of Acidithiobacillus ferrooxidans – ACS Sustainable Resource Management (2025)