How Leaf Arrangement Shapes the Vascular Patterns Inside Fern Stems

A fascinating new study from the University of Tennessee, Knoxville is reshaping what scientists have believed for more than a century about how ferns evolve. Assistant Professor Jacob S. Suissa, from the university’s Department of Ecology and Evolutionary Biology, has discovered that the intricate vascular patterns inside fern stems — those networks that move water and nutrients — are not evolving independently in response to environmental pressures, as previously thought. Instead, they are closely tied to how the leaves are arranged along the stem.
This study, published in Current Biology in October 2025, challenges 150 years of botanical assumptions and offers a clear example of how developmental constraints, rather than direct environmental adaptation, can steer evolutionary outcomes.
What Exactly Was Found
Suissa’s research shows a direct correlation between how many leaves grow around a fern’s stem and how many vascular bundles appear inside it. In simple terms: the pattern outside (the leaves) dictates the pattern inside (the stem’s internal structure).
When Suissa examined various fern species, he found that as the number of leaf ranks increased, so did the number of vascular bundles, often in a near 1:1 ratio. For example, a fern with five vertical ranks of leaves often had five vascular bundles arranged in a corresponding pattern within the stem.
Even more striking was the discovery that where the leaves grow also determines how the vascular bundles are arranged. Ferns with spirally arranged leaves have vascular bundles that form a radial pattern, wrapping around the stem in a circle. But when leaves shift to the dorsal side — the upper part of the stem — something whimsical happens: the cross-section of the stem reveals what looks like a smiley face. This “smiley face” appears because an elongated ventral bundle (on the bottom) is paired with several smaller bundles toward the top, reflecting the clustering of leaves on the upper side.
This observation overturns the long-held belief that vascular structures evolved primarily through natural selection acting directly on internal anatomy. Instead, Suissa’s findings suggest that the arrangement of leaves indirectly governs how the vascular system forms — meaning that one trait’s development constrains and shapes another.
How the Research Was Conducted
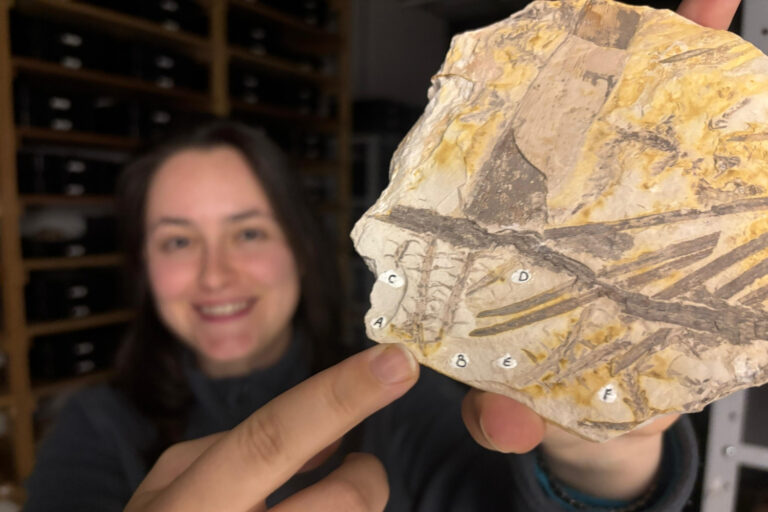
Suissa’s discovery began in the field, during his return to Costa Rica in early 2025. There, he was teaching a Tropical Fern Biodiversity course — the same one he took years earlier as a graduate student. As part of the course, he and his students studied Mickelia nicotianifolia, commonly called the tobacco fern, which has a long, creeping stem that climbs up trees in tropical forests.
While his students focused on data collection, Suissa examined stem samples the old-fashioned way — by cutting them with a razor blade. He noticed that all the leaves grew on the upper (dorsal) side of the stem, and when viewed in cross-section, the internal pattern looked like a smiling face. That observation led him to question whether leaf position might directly influence vascular arrangement.
Back in Knoxville, Suissa expanded the scope of his research. He studied 27 genera of ferns, representing about 30% of global fern diversity. Using microCT imaging — essentially a high-resolution 3D scanning technique similar to a medical CAT scan — he could analyze the internal anatomy of stems without physically slicing them open. This allowed for precise, non-destructive imaging of the internal structures.
Through this advanced imaging and quantitative analysis, Suissa confirmed his field observations: the number and spatial arrangement of leaves on a stem were strongly linked to the number and spatial arrangement of vascular bundles inside it. The relationship was consistent across many genera, demonstrating that this developmental link is a widespread feature of ferns.
Why It Matters
The implications of this research are significant. For over a century, scientists believed that vascular bundles — the tubes transporting water and nutrients — evolved mainly to adapt to environmental conditions such as moisture availability or mechanical support. Suissa’s study challenges that assumption by showing that internal structure can instead be a byproduct of developmental relationships.
In other words, not all traits evolve independently. Some are tied together because of how plants grow and develop. This phenomenon, known as developmental constraint, means that as certain traits (like leaf arrangement) change, others (like stem vasculature) are pulled along for the ride.
Rather than limiting diversity, this developmental linkage can generate new forms. Suissa’s findings illustrate how constraint can fuel innovation — the precise opposite of what many evolutionary biologists might expect. When one trait can’t evolve freely, it sometimes pushes evolution toward creative, unexpected solutions — such as the charming “smiley-face” pattern seen in the tobacco fern.
The Bigger Picture: Ferns and Evolution
Ferns are among the oldest plant lineages on Earth, with a history stretching back nearly 400 million years. They thrived long before flowering plants existed and have maintained remarkable structural diversity through geological time.
Their vascular systems — the networks that transport water, nutrients, and sugars — have been key to their success. These networks form patterns that botanists call steles, and ferns display a wide range of them: from simple ring-shaped types to complex multi-bundled arrangements.
Historically, botanists believed these vascular differences reflected adaptations to varying habitats — for example, thicker stems for drier environments or more bundles for greater flexibility. Suissa’s research adds a new layer: these patterns may not be direct adaptations at all, but rather developmentally inevitable outcomes based on how leaves are spaced.
This insight helps scientists reinterpret fossil ferns as well. When paleobotanists study ancient stem cross-sections, they often try to infer what kind of environment those plants lived in. But if vascular patterns are developmentally linked to leaf arrangement, some of those assumptions might need revision.
How Developmental Constraint Works
In evolutionary biology, developmental constraint refers to the limits imposed on evolution by the way organisms grow and form. Traits that share developmental pathways cannot evolve entirely independently — a change in one inevitably affects the other.
Suissa’s study provides a vivid case study of this principle. The vascular system doesn’t evolve in isolation; it is intertwined with the leaf phyllotaxy — the pattern in which leaves emerge around the stem. Because both are shaped by shared developmental processes, their evolution becomes covaried rather than separate.
This doesn’t mean environment plays no role, but it does mean that evolutionary change is not always about direct adaptation. Sometimes, it’s about what’s developmentally possible given the organism’s internal design.
Tools of Discovery: From Razor Blades to MicroCT Scans
One appealing aspect of Suissa’s work is how it bridges old-school observation and modern technology. The project began with nothing more than a razor blade and a curious mind, but it soon expanded to involve cutting-edge imaging tools and computational evolutionary modeling.
The microCT scanner at UT’s Advanced Microscopy and Imaging Center provided high-resolution 3D images of fern stems, allowing researchers to visualize complex internal anatomy without physically damaging the samples. Combined with quantitative evolutionary tools, Suissa’s team could project anatomical traits backward in time to infer how they evolved across the fern family tree.
What Comes Next
Suissa and his lab are now working on a much larger dataset involving over 300 fern species — representing every fern family known today. By mapping how vascular structures and leaf arrangements have co-evolved across such a massive timescale, the team hopes to understand how different levels of biological construction — from cellular organization to whole-plant architecture — have evolved together.
This work could ultimately refine how we interpret plant evolution more broadly, including in flowering plants and other vascular lineages. It also underscores that observations in the field, even simple ones, can still yield discoveries that challenge long-standing scientific assumptions.
Reference
Research Paper: Fern vascular architecture reveals how developmental constraint can generate novel morphology – Current Biology (2025)