Scientists Discover Why Malaria Parasites Contain Wildly Spinning Iron Crystals
Malaria has been one of humanity’s oldest and deadliest enemies, and researchers have been studying its microscopic culprit—the Plasmodium falciparum parasite—for decades. But even after all that time, some aspects of this tiny organism have remained baffling. One of the strangest mysteries involves the iron crystals that fill every malaria parasite cell. These crystals aren’t still or dormant. They spin, twitch, and ricochet inside the parasite like loose change in a high-speed washing machine. And now, after years of speculation, scientists have finally figured out why they move.
A new study from the University of Utah’s Spencer Fox Eccles School of Medicine, published in the Proceedings of the National Academy of Sciences (PNAS), reveals that the crystals’ motion is powered by a chemical reaction identical to the one that fuels rocket engines. That’s right—the same kind of peroxide breakdown that sends satellites into orbit is happening inside a single-celled parasite in our bloodstream.
The Mysterious Crystals Inside Malaria Parasites
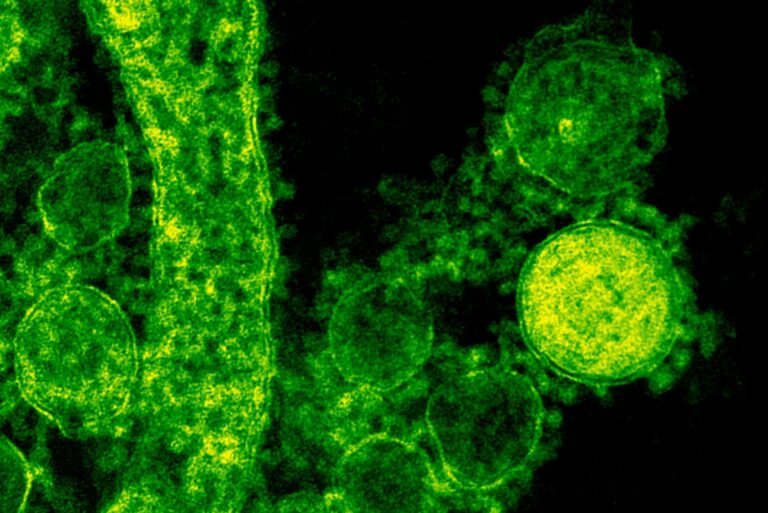
Every Plasmodium falciparum parasite lives inside a red blood cell, feeding on hemoglobin, the molecule that carries oxygen in our blood. As the parasite digests hemoglobin, it’s left with a dangerous by-product: free heme, an iron-based molecule that is highly toxic. To survive, the parasite converts this heme into solid hemozoin crystals, which safely store the excess iron.
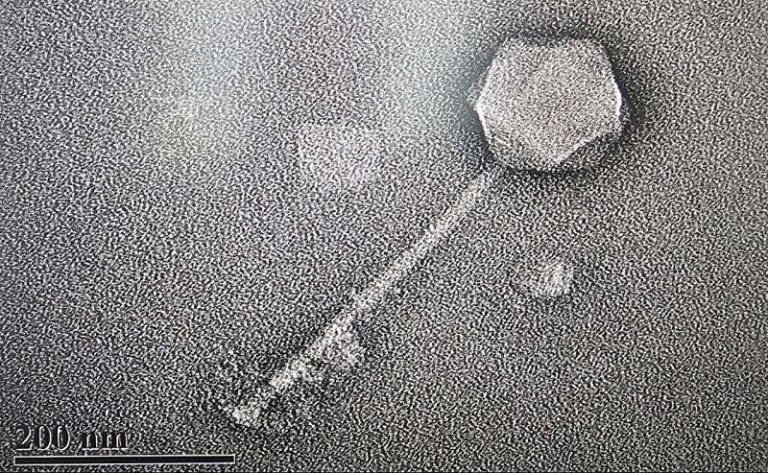
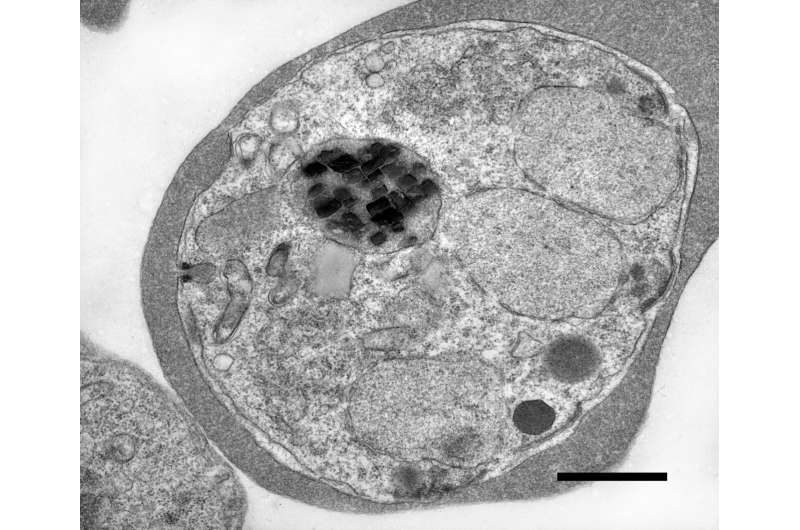
Under an electron microscope, these crystals appear as tiny brick-shaped structures, packed tightly inside a small compartment known as the food vacuole. Scientists have known about hemozoin for decades because it’s a critical part of how malaria parasites detoxify their environment—and it’s also a major target for antimalarial drugs like chloroquine. But until recently, one bizarre fact about these crystals had no explanation: they move on their own.
Researchers observed that as long as the parasite is alive, these iron crystals spin and jolt constantly. Once the parasite dies, the crystals stop moving. The behavior seemed too fast and chaotic to be explained by ordinary thermal motion or vibrations. For years, scientists didn’t talk much about it—because they couldn’t explain it.
A Rocket Reaction in a Red Blood Cell
That mystery changed when biochemist Paul Sigala and his team decided to investigate the source of this motion. They discovered that the crystals are powered by the decomposition of hydrogen peroxide (H₂O₂)—the same reaction used in aerospace propulsion.
Inside the parasite’s food vacuole, hydrogen peroxide naturally accumulates as a waste product of metabolism. When the peroxide comes into contact with the iron surface of the hemozoin crystal, it breaks down into water and oxygen, releasing bursts of energy. Those tiny bursts act like miniature thrusters, giving the crystals the “kick” that makes them spin and bounce around.
This reaction, known in engineering as chemical propulsion, has never before been seen in a biological system. It’s a process humans have used for rockets and submarines, but nature appears to have stumbled upon the same chemistry at the nanoscale.
To test the idea, the researchers placed purified hemozoin crystals in a solution containing hydrogen peroxide—and watched them start to move. No parasite was required. When they reduced the oxygen levels in parasite cultures—thus lowering hydrogen peroxide production—the motion slowed to about half its normal speed, even though the parasites were otherwise healthy.
Why Would the Parasite Want Spinning Crystals?
The discovery of how the crystals move led to a bigger question: why do they move at all? The researchers have a few theories.
One possibility is that the motion helps the parasite detoxify hydrogen peroxide, which is itself extremely harmful. By using the peroxide as a propellant, the parasite may effectively “burn off” the chemical before it can damage its own cells.
Another idea is that the spinning prevents the crystals from clumping together. If the hemozoin particles stuck together, they’d lose surface area, making it harder for the parasite to store additional heme. Constant motion could keep the crystals separate and available for more heme detoxification—an elegant and efficient system for managing waste in such a confined space.
In short, the motion might not just be a side effect—it could be essential for parasite survival.
A First in Biology: Self-Propelled Metallic Nanoparticles
The researchers describe these moving hemozoin crystals as the first known example of self-propelled metallic nanoparticles in a living organism. It’s a finding that bridges biology and physics in a completely unexpected way.
This discovery may also have applications beyond understanding malaria. Scientists are already designing nano-engineered self-propelling particles for medicine and industry—tiny robots that could deliver drugs, clean up pollution, or assemble materials at the molecular level. Studying how malaria parasites naturally achieve nanoscale propulsion could inspire smarter, more energy-efficient designs for artificial micro-robots.
How the Study Was Done
The research team used high-resolution time-lapse microscopy to capture the movement of hemozoin crystals inside living parasites. They tracked each particle frame by frame, measuring changes in brightness and position. The data showed that the crystals’ motion was too fast and too directed to be caused by random Brownian motion.
Next, they ran Brownian dynamics simulations to compare what would happen if the crystals were only jostled by thermal energy. The simulated motion was much slower than what they observed, confirming that an active energy source was involved.
Further experiments revealed that purified crystals could spin on their own in hydrogen peroxide, and that lower oxygen levels—which reduce peroxide production—slowed them down. These combined results strongly supported the chemical propulsion theory.
Interestingly, the malaria parasite’s food vacuole lacks common antioxidant enzymes like catalase, which normally break down hydrogen peroxide. That makes this propulsion mechanism even more fascinating, since it offers a creative way for the parasite to handle a potentially lethal compound without those enzymes.
Why This Discovery Matters for Malaria Research
For decades, scientists have known that drugs like chloroquine and artemisinin work by interfering with hemozoin formation. This new research suggests that targeting the motion and chemistry of hemozoin could be another powerful way to attack the parasite.
If scientists can develop molecules that block the reaction driving the peroxide breakdown, the parasite might not be able to manage its internal chemical stress—and would die. Because human cells don’t rely on this kind of peroxide-driven propulsion, such drugs would likely have fewer side effects.
This discovery also emphasizes how much we still have to learn about malaria’s cell biology. Even after decades of research and millions of lives lost each year, the parasite continues to surprise us with its complexity and adaptability.
Understanding Hemozoin Beyond This Study
Hemozoin has long fascinated scientists, not just because of its role in malaria but because it’s one of the few examples of a biologically produced crystal made entirely of metal-based molecules. It’s magnetically active, light-sensitive, and highly ordered—qualities that have inspired new ideas for diagnostics and even biomaterials research.
For example, scientists are exploring whether the magnetic properties of hemozoin can be used for noninvasive malaria detection using laser or magnetic resonance techniques. The more we understand about how hemozoin behaves—including its surprising motion—the more potential applications we might unlock.
A Blend of Rocket Science and Parasitology
This discovery is remarkable not just because it solves a decades-old mystery, but because it connects two seemingly unrelated worlds: rocket science and parasitology. The same reaction that launches spacecraft is quietly happening inside one of the most deadly microbes on Earth.
It’s a powerful reminder that nature often discovers elegant solutions to complex problems long before humans do. By studying the strange, energetic dance of these microscopic iron crystals, scientists may not only find new ways to fight malaria but also uncover principles that shape the future of nanotechnology.
Research Reference:
Chemical propulsion of hemozoin crystal motion in malaria parasites – Proceedings of the National Academy of Sciences (2025)