Scientists Discover That Some Exoplanets Can Create Their Own Water
A new study published in Nature has offered a fascinating explanation for a long-standing cosmic mystery — how certain exoplanets appear to have an abundance of water even when they orbit too close to their stars for ice to exist. The research suggests that these planets may literally make their own water deep within their interiors, through reactions between molten rock and hydrogen gas under immense pressure.
Understanding the Mystery of Water-Rich Exoplanets
Astronomers have found thousands of exoplanets across the galaxy, and among them, a puzzling class stands out: sub-Neptunes. These are planets between the size of Earth and Neptune. They typically have a rocky core surrounded by an envelope of either hydrogen gas or water. That makes sense for planets forming far from their host stars — regions where water can exist as ice and accumulate during planet formation.
But some of these sub-Neptunes orbit much closer to their stars, where it should be far too hot for water to survive. What’s more, the amount of water they contain is much greater than what could plausibly come from comets or asteroids. So, how can these planets be so wet?
The Surprising Answer: Water From Within
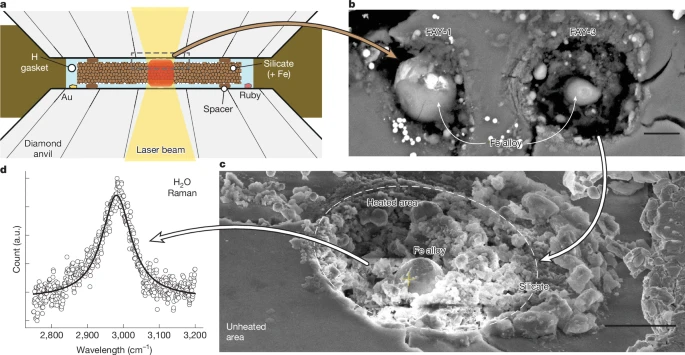
The new study, titled “Building Wet Planets Through High-Pressure Magma–Hydrogen Reactions” (Horn et al., 2025), proposes a groundbreaking explanation. The authors conducted a series of laser-heated diamond-anvil cell experiments, which recreate the extreme pressure and temperature conditions inside exoplanets.
In these experiments, they placed silicate rock samples — minerals like olivine and fayalite — into a dense hydrogen atmosphere, then subjected them to pressures up to 42 gigapascals (around 400,000 times Earth’s atmospheric pressure) and temperatures reaching 3,900 Kelvin. Under those conditions, the rock melted into magma, and the oxygen within the silicate minerals was freed to react with hydrogen, forming water (H₂O).
The results were astonishing. The reactions produced water concentrations up to 18% by weight in the experimental samples — thousands of times higher than previous models predicted. This means that planets with hydrogen-rich atmospheres and molten interiors could be manufacturing large amounts of water from within.
A Chemical Transformation on Planetary Scales
Here’s what this means in planetary terms: in hydrogen-rich sub-Neptunes, the boundary between the rocky core and the hydrogen atmosphere is subject to extreme pressures and temperatures. At that boundary, hydrogen atoms can diffuse into molten silicate rock and trigger chemical reactions that pull oxygen out of the rock, creating silicon alloys and magnesium oxides — and producing water as a byproduct.
This process could continue as long as there’s molten rock and hydrogen available to react. Over time, the planet’s hydrogen envelope would thin out as hydrogen gets converted into water, shifting the planet from a hydrogen-dominated world to a water-rich one. Essentially, hydrogen-rich sub-Neptunes could evolve into water worlds or super-Earths.
This also suggests that “Hycean worlds” — planets with a hydrogen-rich atmosphere overlying a deep ocean — may be far more common than previously thought.
Rethinking How Planets Form
Before this research, scientists assumed that water-rich planets had to form beyond the “snow line”, the region in a solar system where temperatures are cold enough for water to condense into ice. After formation, such planets might migrate inward, carrying their icy material closer to their star.
However, this new study challenges that traditional view. It implies that even planets forming close to their stars, where it’s too hot for ice, could still become water-rich thanks to internal chemistry. This revelation widens the possibilities for where habitable environments could exist in the galaxy.
It also changes how astronomers interpret observations of exoplanet atmospheres. When water vapor is detected, it might not always mean that the planet came from a cold, icy region — it could mean that the planet made its own water.
Inside the Experiments: How Scientists Simulated Alien Worlds
The team used diamond-anvil cells, instruments capable of creating enormous pressures by squeezing tiny samples between two diamonds. Lasers were used to heat the samples to mimic the interior of a sub-Neptune planet.
The rock samples (such as San Carlos olivine, fayalite, and silica mixed with iron) were placed in a hydrogen-rich environment. During the reaction, the silicate structure broke down, freeing oxygen atoms that combined with hydrogen to form water. The resulting byproducts included iron-silicon alloys and magnesium oxide, confirming that oxygen had indeed left the rock to bond with hydrogen.
These experiments revealed that water can form directly inside the planet’s mantle region under the right conditions — not just on the surface or in the atmosphere.
Why This Matters for Habitability
Water is one of the key ingredients for life as we know it. The discovery that planets can generate their own water greatly expands the number of worlds that could potentially be habitable. Planets that were previously thought to be too close to their stars, and therefore too hot and dry, might in fact harbor vast internal oceans.
That doesn’t mean these worlds are automatically friendly to life — factors like temperature, radiation, and chemical balance also play crucial roles — but it does suggest that liquid water could be far more common than we ever imagined.
Connecting Hydrogen-Rich and Water-Rich Planets
The researchers propose that hydrogen-rich sub-Neptunes and water-rich sub-Neptunes aren’t entirely different kinds of planets. Instead, they represent different stages of evolution.
A young planet may start with a thick hydrogen envelope. Over time, hydrogen interacts with molten rock in the interior, forming water and gradually consuming much of the hydrogen. As a result, the planet’s composition shifts toward being water-rich, and if it loses most of its remaining hydrogen to space, it could end up as a super-Earth — a rocky planet with oceans or deep water layers.
This also helps explain the continuum observed among exoplanets, from hydrogen-heavy to water-heavy atmospheres, rather than distinct categories.
The Bigger Picture: What Comes Next
The discovery opens up many new research directions. Future experiments will likely explore different planetary materials — not just silicates but also other minerals and elements that could influence water formation. Scientists also hope to study a wider range of pressure and temperature conditions to see how robust this water-making process really is.
Observational astronomers, meanwhile, can use these findings to refine how they interpret exoplanet atmospheres. When the James Webb Space Telescope or future observatories detect water signatures, researchers will now have to consider whether the water came from internal chemistry rather than external sources.
Open Questions and Ongoing Challenges
While this study provides compelling experimental evidence, there are still open questions. For example:
- How efficiently can hydrogen penetrate deep into a planet’s molten layers?
- How much water can realistically accumulate before the reaction slows down?
- What happens to the water — does it stay trapped in the mantle, or does it migrate to the surface?
- How long can a planet maintain the conditions needed for these reactions?
Understanding these details will help scientists build more accurate models of planetary interiors and evolution.
A Universe Full of Wet Worlds?
If this process is common, then water-rich planets may be far more abundant than we ever thought. The galaxy could be full of worlds that didn’t need icy beginnings to become blue and wet. In other words, the conditions for potential habitability might not be confined to the distant, cold reaches of solar systems.
This realization reshapes our understanding of planet formation — and it makes the universe feel just a little more alive with possibilities.
Reference:
Building Wet Planets Through High-Pressure Magma–Hydrogen Reactions (Nature, 2025)