Scientists Turn Bacteria’s Own Genes Against It to Tackle Antibiotic Resistance
A research team at St. Jude Children’s Research Hospital has found a groundbreaking way to fight back against one of the most stubborn antibiotic-resistant bacteria on the planet—Mycobacterium abscessus. This pathogen, often called the “antibiotic nightmare” in medical circles, is notoriously difficult to treat, especially in people with lung disease or weakened immune systems. The scientists have developed a modified version of the antibiotic florfenicol, known as florfenicol amine (FF-NH₂), which cleverly uses the bacterium’s own resistance genes to destroy it.
This study, published in Nature Microbiology in October 2025, introduces a new concept in antibiotic research called resistance hacking—a strategy that doesn’t just bypass bacterial defenses but turns them into weapons against the microbe itself.
The Antibiotic Resistance Crisis
Antibiotic resistance is one of the most serious threats to global health today. Bacteria have evolved numerous ways to survive even the most powerful drugs—through enzymes that break antibiotics down, pumps that eject them, or genetic regulators that turn on defense systems.
Mycobacterium abscessus is particularly notorious for this. It’s a rapidly growing non-tuberculous mycobacterium (NTM) found in soil and water and has become a growing problem in hospitals and clinics worldwide. For people with conditions like cystic fibrosis, bronchiectasis, or hematological cancers, this pathogen can lead to long-lasting and often deadly infections.
The bacterium’s resistance comes mainly from a complex genetic network controlled by a regulator called WhiB7. This gene acts like a “command center” that activates more than 100 resistance-related proteins whenever antibiotics attack. These proteins include acetyltransferases, efflux pumps, and methyltransferases that protect the ribosome—the main antibiotic target in mycobacteria. Because of this powerful system, even aggressive antibiotic treatments fail, leaving patients with limited options and long, toxic treatment courses.
A Clever Twist: Using Resistance to Trigger Self-Destruction
The St. Jude team, led by Dr. Richard Lee and Dr. Gregory Phelps, was originally studying analogs of chloramphenicol and florfenicol—two older antibiotics that target the bacterial ribosome. While modifying florfenicol’s chemical structure, they noticed something surprising.
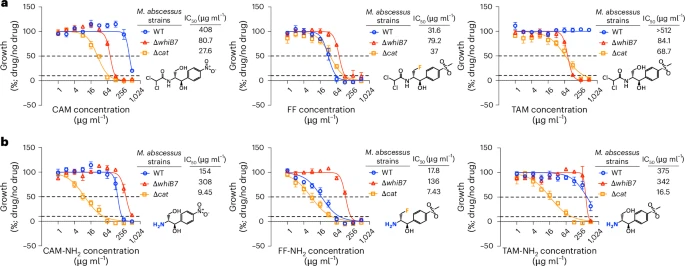
The engineered compound FF-NH₂ worked strongly against normal strains of M. abscessus but failed against strains where the WhiB7 gene was deleted. This was the opposite of what normally happens. Usually, knocking out a resistance gene makes bacteria more vulnerable, not more resistant. That clue led the team to dig deeper.
They discovered that FF-NH₂ acts as a prodrug—a harmless precursor that becomes active only inside the bacterium. Once inside, the bacterium’s own resistance enzyme, called Eis2, converts FF-NH₂ into its active antibiotic form through an acetylation reaction. This conversion process is driven by the WhiB7 regulon itself, meaning the bacteria literally activate the very drug that kills them.
Even more fascinating, this sets up a self-amplifying feedback loop:
- FF-NH₂ enters the bacterium in an inactive form.
- The bacterium’s WhiB7 system detects ribosomal stress and produces more Eis2 enzymes.
- Eis2 enzymes activate more FF-NH₂ molecules into their potent form.
- The active drug further stresses the ribosome, keeping WhiB7 switched on.
This circular activation process keeps the antibiotic’s effect going perpetually, ensuring that the bacterium’s resistance machinery keeps fueling its own destruction.
Why This Approach Is Safer
Traditional antibiotics, especially those in the phenicol family, can cause mitochondrial toxicity in human cells. Since mitochondria are evolutionary descendants of bacteria, some antibiotics that target bacterial ribosomes also disrupt mitochondrial protein synthesis. This can lead to hearing loss, fatigue, and organ damage with long-term treatment.
Because florfenicol amine stays inactive until it meets the bacterial enzyme, it avoids damaging human mitochondria. The compound is also narrow-spectrum, meaning it specifically targets M. abscessus and closely related species like M. chelonae, while leaving most of the beneficial microbiome untouched.
This targeted precision could finally offer a safer, more tolerable therapy for patients who need months or even years of antibiotic treatment.
Proof of Concept and Experimental Details
In lab experiments, the team used mutant bacterial strains that lacked either WhiB7 or the Eis2 enzyme. In these mutants, the prodrug completely lost its effectiveness, confirming that the drug relies on these resistance mechanisms to work.
They also performed RNA sequencing and proteomic analysis to track which genes were activated after treatment. The results showed strong upregulation of the WhiB7-controlled resistance network, including Eis2, validating the feedback loop model.
In mouse infection models, treatment with FF-NH₂ led to reduced bacterial loads in the lungs, liver, and spleen compared with untreated controls. While early, these findings suggest that the drug can work in living organisms, not just in test tubes.
Implications for Future Antibiotic Design
This discovery goes beyond one bacterium. It introduces a whole new way of thinking about antibiotic resistance. Instead of fighting resistance genes, scientists could harness them. By designing drugs that are activated by bacterial enzymes, we could turn resistance mechanisms into Achilles’ heels rather than shields.
The concept could potentially be extended to other pathogens that express unique resistance proteins, such as Pseudomonas aeruginosa or Acinetobacter baumannii. With advances in structural biology and data science, researchers could screen for bacterial enzymes that might serve as natural “switches” for new prodrug candidates.
This kind of pathogen-specific antibiotic design also aligns with the global push for precision antimicrobials—drugs that eliminate the target pathogen without harming the microbiome or driving broad resistance.
The Long Road Ahead
While this new approach looks extremely promising, it’s still in the preclinical stage. There have been no human trials yet, and safety, dosing, and pharmacokinetics must all be studied thoroughly. Even though the compound avoids many toxic effects seen in earlier phenicols, researchers will need to confirm its safety in larger animals and eventually in humans.
Another challenge is drug resistance evolution. The study found that mutants lacking WhiB7 or Eis2 could survive FF-NH₂ treatment. Although these mutants may grow more slowly or be less virulent, the possibility of resistance developing over time can’t be ignored. Combining this new antibiotic with existing therapies or cycling different drugs might help prevent resistance from taking hold.
On the practical side, bringing any new antibiotic to market is difficult and expensive. The economic model for antibiotic development is still broken—most pharmaceutical companies are hesitant to invest in drugs that are meant to be used sparingly. For this discovery to reach hospitals, partnerships between academic labs, government agencies, and biotech firms will be essential.
Understanding Mycobacterium abscessus
To fully appreciate the impact of this research, it helps to know why M. abscessus is so tough. Unlike many bacteria, it has a thick, waxy cell wall rich in mycolic acids, which makes it impermeable to most antibiotics. It also grows relatively slowly, allowing it to hide from immune responses and resist treatment.
In hospitals, M. abscessus infections often occur after surgery, catheter use, or inhalation in patients with preexisting lung damage. Standard treatment involves a cocktail of strong antibiotics such as amikacin, clarithromycin, and cefoxitin, sometimes for over a year. Side effects are common, and cure rates are frustratingly low.
That’s why this discovery matters. If florfenicol amine lives up to its promise, it could become the first targeted, low-toxicity therapy for these hard-to-treat infections.
What Comes Next
The St. Jude team plans to explore whether this “resistance-hijacking” approach can be adapted to other pathogens. They’re also looking into optimizing the drug’s structure and studying its potential use in combination therapies.
If successful, this could mark a turning point in antibiotic research—a move away from the endless arms race against resistance and toward smarter, self-activating drugs that flip bacterial defenses inside out.
For now, the idea that we can make bacteria unwittingly activate their own killers is both ingenious and hopeful. It’s a reminder that even in the face of antibiotic resistance, science still has some creative tricks up its sleeve.
Research Reference: Phelps G. A. et al., Prodrug florfenicol amine is activated by intrinsic resistance to target Mycobacterium abscessus, Nature Microbiology (2025)