How Leucine Boosts Mitochondrial Energy Production by Protecting Key Proteins
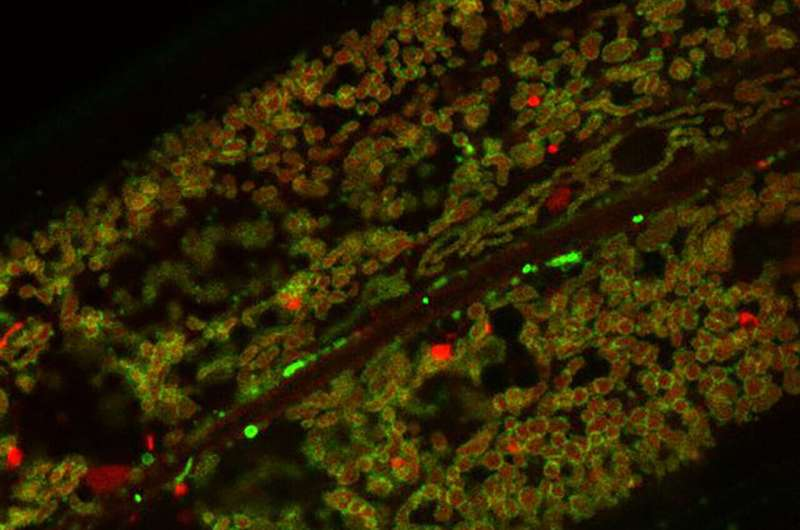
Mitochondria are often called the powerhouses of the cell because they generate the energy that keeps every biological process running — from muscle movement to brain activity. But the inner workings of these organelles are far from static. They constantly adjust to the cell’s energy needs, responding to stress, exercise, diet, and disease. What scientists have long wondered, however, is how nutrients in our food directly influence these adjustments. A recent study published in Nature Cell Biology has finally revealed one clear mechanism, and it revolves around a familiar amino acid — leucine.
A research team led by Professor Thorsten Hoppe from the University of Cologne’s Institute for Genetics and the CECAD Cluster of Excellence on Aging Research has discovered that leucine helps mitochondria function more efficiently by preventing the degradation of certain outer membrane proteins. These proteins are crucial for importing molecules that mitochondria need to produce energy. By protecting them, leucine effectively enhances mitochondrial respiration, allowing cells to make energy faster and more efficiently.
Leucine’s Essential Role in the Body
Leucine is one of the nine essential amino acids, which means our bodies can’t produce it — we have to get it through diet. It’s found in protein-rich foods such as meat, dairy products, and legumes like beans and lentils. Apart from being a building block for proteins, leucine has long been known for its role in muscle repair and growth, which is why it’s a common ingredient in many fitness supplements.
But this new research shows that leucine’s benefits go well beyond muscles. It doesn’t just help build tissue; it actually regulates how our cells make and manage energy. This makes leucine one of the most metabolically powerful nutrients we consume.
The Discovery: Protecting the Mitochondrial Surface
The study focused on the outer mitochondrial membrane (OMM) — the boundary layer that controls what enters and exits the mitochondria. This membrane contains proteins that act as transport channels, moving molecules necessary for energy production into the organelle.
The researchers found that leucine prevents these OMM proteins from being broken down. Normally, the cell uses a process called ubiquitin-mediated degradation to remove damaged or excess proteins. While this is essential for maintaining protein quality, it can sometimes degrade useful proteins if not properly regulated.
Leucine, as it turns out, can stabilize these outer membrane proteins by reducing their degradation. This means that more of these proteins remain intact and functional, allowing mitochondria to import metabolic molecules more effectively. As a result, cellular energy production increases.
The Key Players: SEL1L and GCN2
To understand how leucine achieves this protective effect, the researchers identified two important proteins involved in the process: SEL1L and GCN2.
SEL1L acts as part of the cell’s quality control system. It helps identify and target misfolded or damaged proteins for destruction. However, when leucine levels are high, SEL1L activity decreases, which slows down the breakdown of mitochondrial surface proteins.
This suppression of SEL1L is controlled by GCN2, an amino acid sensor that detects when nutrients are scarce. When leucine is abundant, GCN2 becomes less active, leading to lower SEL1L activity. This creates a cascade effect — less SEL1L means less protein degradation, allowing mitochondria to keep more of their functional outer membrane proteins intact.
By revealing this leucine → GCN2 → SEL1L pathway, the study uncovers a new way that diet and metabolism are linked directly to mitochondrial efficiency.
Testing the Theory in Worms and Human Cells
To see if this mechanism held true across species, the team tested it in the roundworm Caenorhabditis elegans, a model organism often used in aging and metabolic research. They discovered that worms with defects in leucine breakdown had impaired mitochondrial function and even reduced fertility.
In human lung cancer cells, they observed a related phenomenon. Mutations that interfere with leucine metabolism caused an increase in intracellular leucine levels, which in turn stabilized mitochondrial proteins. This stabilization made the cancer cells more resistant to stress and enhanced their survival.
These findings highlight both the potential and the caution needed in manipulating leucine pathways — what’s beneficial for normal cells could also make cancer cells more resilient.
What This Means for Health and Disease
The discovery that leucine can directly regulate mitochondrial performance opens new doors for treating diseases linked to low energy production, such as metabolic disorders, mitochondrial diseases, and certain neurodegenerative conditions. By understanding how leucine affects SEL1L and mitochondrial stability, scientists could potentially develop therapies that mimic or enhance this effect.
However, the researchers also emphasize caution. While boosting leucine levels can improve mitochondrial efficiency, SEL1L’s role in removing damaged proteins is equally vital. Suppressing this process too much could lead to the accumulation of faulty proteins, which in the long run might harm cells instead of helping them.
This balance between maintaining energy output and ensuring protein quality is delicate — and understanding it better could be key to both longevity and metabolic health.
Beyond the Study: Why Mitochondria Matter So Much
Mitochondria do more than just produce energy. They are deeply involved in cellular aging, immune response, and programmed cell death (apoptosis). Their dysfunction has been linked to numerous diseases, from diabetes and Parkinson’s disease to muscular dystrophy and infertility.
Maintaining healthy mitochondria is one of the most important aspects of keeping our bodies functioning efficiently. Exercise, proper sleep, and balanced nutrition all support mitochondrial health, but this study shows that specific nutrients like leucine can play a far more active role than we once thought.
The discovery also connects to a broader field of study known as nutrient signaling, where researchers investigate how food components act as biochemical messengers rather than just fuel. Leucine, for instance, is already known to activate a pathway called mTOR, which regulates cell growth and metabolism. Now, it appears to also play a direct role in mitochondrial adaptation, expanding our understanding of how diet shapes cellular function.
The Potential for Future Research
This finding raises many new questions. Could leucine supplements enhance mitochondrial function in people with metabolic fatigue? How might different levels of dietary leucine affect longevity or athletic performance? And most importantly, could targeting the leucine–SEL1L axis provide a way to restore energy production in diseases where mitochondria fail?
The answers will require careful studies, especially because cancer cells might exploit this same mechanism to survive. Still, by mapping out this pathway, scientists have a new framework to explore how nutrition and cellular energy are connected.
A Nutrient With Dual Power
In simple terms, this study redefines leucine as more than just a muscle nutrient. It’s a metabolic signal that helps cells decide how to manage their energy resources. By keeping mitochondrial surface proteins stable, leucine gives cells the ability to ramp up energy production when nutrients are abundant — a kind of “fuel efficiency mode” built into our biology.
This balance between nutrient sensing, protein quality control, and mitochondrial adaptation could hold the key to understanding not only how our cells stay healthy but also how they age and respond to disease.
For now, the takeaway is clear: what we eat doesn’t just feed us — it instructs our cells how to function. And in the case of leucine, it seems to be telling our mitochondria to keep running strong.
Research Reference:
Leucine inhibits degradation of outer mitochondrial membrane proteins to adapt mitochondrial respiration – Nature Cell Biology (2025)