Bowhead Whales’ Extraordinary Longevity Linked to a Powerful DNA-Repair Protein Called CIRBP

Bowhead whales are already famous for their size and their serene existence beneath Arctic waters, but scientists have now discovered a molecular secret that might explain how these whales live more than 200 years while staying remarkably healthy. A new study published in Nature by researchers at the University of Rochester reveals that bowhead whales have incredibly high levels of a protein called CIRBP (cold-inducible RNA-binding protein), which appears to play a crucial role in repairing damaged DNA — a process central to longevity and cancer resistance.
The Bowhead Whale and Its Mystery of Longevity
The bowhead whale (Balaena mysticetus) is the longest-living mammal known, with some individuals estimated to be over 200 years old. Despite their immense size — reaching up to 60 feet in length and weighing around 100 tons — they show remarkably low rates of cancer and age-related disease. This challenges what’s known as Peto’s Paradox, the biological puzzle that larger animals with more cells (and more opportunities for mutations) don’t necessarily have higher cancer risks. For decades, scientists have wondered how these whales avoid the fate of smaller, shorter-lived species that develop diseases with age.
The new study, titled “Evidence for improved DNA repair in long-lived bowhead whale”, delves deep into this paradox. The findings provide compelling evidence that bowhead whales have evolved exceptional cellular mechanisms to maintain genome integrity over their long lifespans — and that CIRBP might be the key.
What the Study Found
The team, led by Professors Vera Gorbunova and Andrei Seluanov from the University of Rochester, worked with postdoctoral researcher Denis Firsanov and graduate student Max Zacher to study whale cells and compare them with those of other mammals, including humans, mice, and cows. Their analysis revealed that bowhead whale cells are significantly better at repairing double-strand breaks in DNA — one of the most dangerous forms of genetic damage.
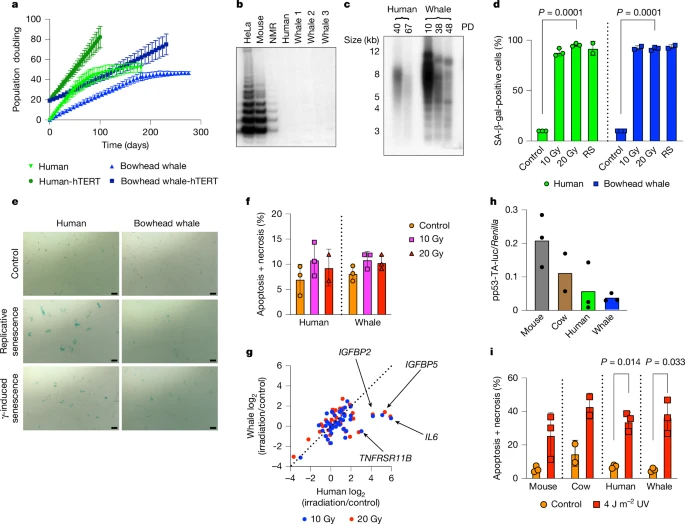
b, Telomerase activity in skin fibroblasts from mouse, naked mole rat, human, and three bowhead whales (1–3) measured by TRAP assay; HeLa cells used as positive control.
c, Telomere length in human and bowhead whale fibroblasts at indicated population doublings (PD) measured by TRF assay (see Supplementary Fig. 1).
d, Percentage of SA-β-gal-positive fibroblasts after γ-irradiation (12 days, n = 3 per species) or during replicative senescence (RS, n = 2); Welch’s two-sided t-test.
e, Representative SA-β-gal staining images after γ-irradiation or RS (scale = 100 µm).
f, Cell death quantified by annexin V/PI assay 3 days after γ-irradiation (n = 3 per species).
g, SASP gene expression 12 days after γ-irradiation in human and whale fibroblasts.
h, p53 reporter activity in fibroblasts from mouse, cow, human, and whale transfected with a p53-responsive luciferase vector (n = 3 for mouse, human, whale; n = 2 for cow).
i, Cell death after UVC irradiation measured by annexin V/PI assay (n = 3 for mouse, human, whale; n = 2 for cow); Welch’s two-sided t-test. Data are mean ± s.d.
Credit: Nature (2025)
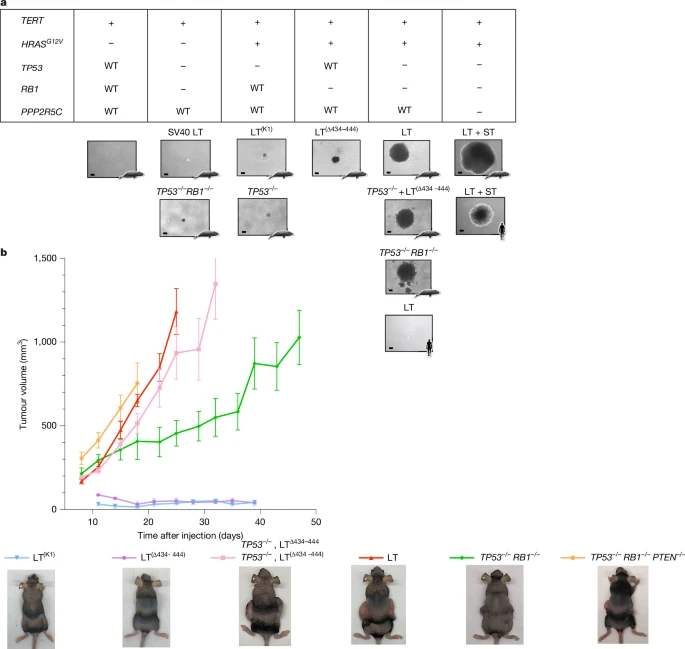
b, Tumour growth curves of bowhead whale fibroblast lines in mouse xenografts. All lines express HRAS(G12V) and hTERT, with indicated genotypes. Each point represents six injections per line (12 for TP53−/−RB1−/− double knockouts). Experiments ended at maximum tumour size or time limit; representative endpoint images shown. Error bars, s.e.m.
Credit: Nature (2025)
When DNA strands break, cells can repair them in a few different ways, such as through homologous recombination (HR) or non-homologous end joining (NHEJ). These processes are vital for preventing harmful mutations that can lead to cancer. The whale cells outperformed all other species tested, repairing breaks faster and more accurately than human cells could.
To quantify this, the researchers exposed whale fibroblast cells to DNA-damaging conditions and found far fewer mutations, fewer insertions or deletions, and less structural damage than in other mammalian cells. This suggests that the bowhead whale’s biology has fine-tuned its repair systems to preserve genetic stability over an exceptionally long lifetime.
CIRBP: The Star of the Study
Among all the DNA-repair-related proteins identified in the whale’s cells, CIRBP stood out. The bowhead whales had around 100 times more CIRBP than other mammals. CIRBP is known as a cold-inducible protein, meaning it increases in response to lower temperatures — which makes sense for an Arctic species living in icy waters.
When the research team took the whale version of the CIRBP gene and introduced it into human cells and fruit fly cells, the results were striking. DNA repair efficiency in these cells improved substantially, and fruit flies expressing the whale’s CIRBP even lived longer than normal flies. The effect was consistent: whether in whales, humans, or flies, higher CIRBP activity meant stronger defense against DNA damage.
This protein appears to act as a guardian of the genome, stabilizing RNA molecules and supporting DNA repair pathways when cells are stressed by cold or other environmental challenges. In whale cells, this elevated activity may have evolved as a survival mechanism that also happens to delay aging and prevent cancer.
How Cold Affects CIRBP Levels
An intriguing detail of the study is how temperature influences CIRBP production. When researchers slightly lowered the temperature in mammalian cell cultures, they observed that the cells began producing more CIRBP protein. For bowhead whales, living in the freezing Arctic may constantly keep CIRBP levels elevated, ensuring that DNA repair processes stay highly active.
The scientists suggest that in humans, cold exposure — such as cold showers or cryotherapy — might theoretically stimulate CIRBP production as well, though this idea is still purely speculative. It’s not yet clear how much cold exposure would be necessary or whether it would yield the same DNA-protective effects in people.
Cancer Resistance and Genome Maintenance
The study also addressed how bowhead whales deal with cancer formation. Under the multi-stage model of cancer, most human tumors arise after five to seven harmful genetic “hits” accumulate in key genes controlling cell division and DNA repair. Based on this model, one might expect that whales — with vastly more cells and centuries-long lifespans — would be far more prone to cancer. But the opposite is true.
When the researchers tested how many “oncogenic hits” it took to turn whale cells cancerous, they were surprised to find fewer hits were required compared to humans. That means the whales don’t rely on needing many mutations to resist cancer — instead, their cells are simply less likely to accumulate mutations in the first place. In other words, whales prevent damage rather than just resisting its effects.
This is a subtle but powerful difference. While other large animals, such as elephants, have evolved multiple copies of tumor-suppressor genes like TP53, bowhead whales appear to depend on hyper-efficient DNA repair systems to maintain their health over time.
What This Could Mean for Humans
The implications of this discovery extend far beyond marine biology. If scientists can find ways to safely enhance CIRBP activity in humans, we might one day be able to improve DNA repair, resist cancer, and slow aging. Researchers are exploring two main possibilities: boosting our body’s natural CIRBP production, or developing therapies to deliver additional CIRBP directly to cells.
Although it’s far too early to apply this in humans, the research highlights how much can be learned from long-lived species. Studying nature’s most resilient creatures — whales, bats, turtles, naked mole rats — is helping scientists uncover the genetic and cellular strategies that make long life possible.
The team is also planning follow-up experiments in mice to see if higher CIRBP expression can improve DNA repair and increase lifespan in mammals more similar to humans.
The Bigger Picture: Why DNA Repair Matters
DNA repair is at the heart of healthy aging. Every day, the DNA inside our cells suffers thousands of breaks from metabolic by-products, UV radiation, and environmental toxins. Efficient repair mechanisms prevent this damage from accumulating. As we age, these systems naturally slow down, leading to mutations, cancer, and cellular dysfunction.
By understanding how species like the bowhead whale maintain strong genome maintenance for centuries, researchers can design interventions to mimic those benefits. This could include not only genetic or protein-based therapies but also lifestyle practices that support DNA stability — from diet and stress reduction to possibly even temperature-based approaches that activate cold-inducible pathways.
Why Bowhead Whales Are So Special
Bowhead whales live exclusively in Arctic and sub-Arctic waters, enduring freezing temperatures and crushing sea ice. They’ve adapted to this harsh environment with a thick layer of blubber, a slow metabolism, and an immune system fine-tuned for longevity. Their heart rate slows dramatically during dives, reducing oxygen stress, and their cells operate at a low metabolic rate — all factors that may reduce oxidative damage to DNA.
Unlike some animals that rely on extreme metabolic or regenerative adaptations, bowhead whales appear to have taken a genetic route to long life, evolving superior repair and maintenance systems rather than rapid regeneration or frequent cell turnover. Their biology demonstrates that longevity doesn’t just come from avoiding disease, but from continuously fixing what goes wrong at the molecular level.
The Takeaway
The discovery of the role of CIRBP in bowhead whale longevity provides a fascinating window into the biology of aging. It suggests that living long and staying healthy isn’t just about avoiding damage but about repairing it better than anyone else in the animal kingdom. For humans, this could mean that the key to extending lifespan lies not in miracle drugs, but in learning from nature’s best engineers — the whales that thrive for centuries under the Arctic ice.
Research Paper: Evidence for improved DNA repair in long-lived bowhead whale – Nature (2025)