Scientists Build Functional Calcium Channels Entirely From Scratch Using AI
A team of researchers at the University of Washington’s Institute for Protein Design has achieved something remarkable — they’ve created working calcium ion channels completely from scratch, without copying nature’s designs. This achievement marks one of the first times that such complex biological machinery has been built bottom-up using artificial intelligence (AI) and computational protein design. The work, led by Yulai Liu, a postdoctoral scholar in David Baker’s lab, was recently published in Nature.
These new calcium channels are engineered proteins that mimic how natural ion channels regulate electrical signaling in cells — the very process behind nerve impulses, muscle contraction, and heartbeat rhythms. The breakthrough demonstrates that scientists can now design highly specific, membrane-embedded proteins capable of precise ion selectivity and function — something that even decades of biochemistry research had struggled to fully replicate.
What Are Ion Channels and Why They Matter
Ion channels are tiny molecular pores embedded in the membranes of living cells. They act like electrical gates, opening and closing to allow specific charged particles, or ions, to flow in and out. These channels are essential to life. They’re responsible for neurons firing, heartbeats pulsing, and muscles contracting.
Among these, calcium channels play a special role. When calcium ions enter cells through these channels, they act as signals to trigger important biological events such as the release of neurotransmitters in neurons or the contraction of muscle fibers. Natural calcium channels are extremely selective — they allow calcium ions through while blocking others like sodium — and that selectivity is vital for healthy cellular function.
However, studying these natural channels is difficult. They’re complex, delicate, and deeply embedded in cellular membranes. That’s where this new work comes in: instead of trying to tweak existing channels, the team decided to design new ones from the ground up.
Designing Ion Channels from First Principles
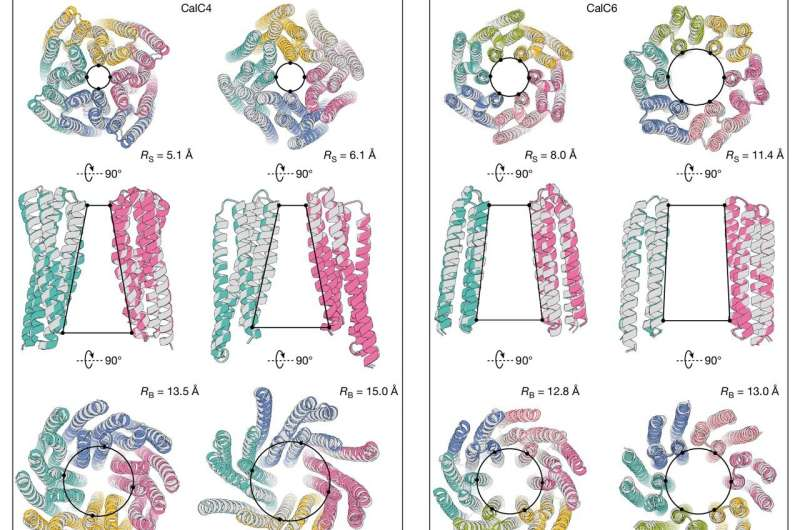
The researchers began by focusing on the selectivity filter — the narrowest part of the channel that determines which ions can pass through. Using RFdiffusion, an AI-based protein design tool, they specified the geometry that would favor calcium ions (Ca²⁺) while blocking others.
This structure was used as a starting point. From there, the AI built out the rest of the protein — the supporting structure, transmembrane regions, and the entire pore — ensuring that the resulting molecule could fold correctly and function inside the lipid membrane of a cell.
This approach is quite different from modifying existing proteins. Instead of nature providing the blueprint, the AI and researchers defined exact atomic arrangements to produce a completely new molecule that behaves like a natural one.
To ensure stability and proper assembly, the designed channels were made symmetrical, typically forming four or six identical subunits (called tetramers or hexamers). This symmetry helps maintain a uniform pore structure, much like the architecture found in natural channels.
Once the designs were finalized, the team expressed them in insect cells — a common system for producing and studying complex proteins — and began testing whether they worked.
Testing the Artificial Channels
To find out if their synthetic calcium channels actually functioned, the researchers used a technique called patch-clamp electrophysiology. This method measures electrical currents passing through ion channels when they open and close.
Several of the designed channels successfully conducted calcium ions, while blocking sodium ions as intended. In fact, the channels transmitted roughly five times more current for calcium than for sodium, demonstrating clear ion selectivity — a defining property of natural calcium channels.
But the verification didn’t stop there. The researchers also used cryo-electron microscopy (cryo-EM) to take high-resolution 3D images of the channels. In one striking case, the experimentally observed structure matched the computational model with atomic precision. This result confirmed that the AI’s design process had predicted not only the correct function but also the exact physical structure of the protein.
The Science Behind the Breakthrough
Building a membrane protein from scratch is one of the toughest challenges in biology. Most proteins catalogued in structural databases are water-soluble, floating freely inside cells. Ion channels, however, must embed themselves into the cell’s lipid membrane, a greasy, two-layered barrier that separates the cell’s interior from the outside world.
Designing for that environment means crafting sequences of amino acids that are both hydrophobic enough to remain stable within the membrane and precisely folded to create a pore. This balancing act has long made ion channel design nearly impossible.
By combining deep learning–based protein modeling and generative design algorithms, the UW team overcame these challenges. The AI system RFdiffusion allowed them to explore a vast space of possible structures until they identified those that could form stable, selective calcium channels.
This work extends beyond simply making a functioning protein. It shows that even biochemical systems not fully understood by humans can be reconstructed from first principles when guided by AI models and physical constraints.
Implications for Biology and Medicine
The potential applications of this work are enormous. Synthetic ion channels could serve as powerful tools for:
- Neuroscience, allowing researchers to precisely control electrical signaling in nerve cells.
- Cardiology, by modeling heart rhythm disorders and testing new drugs.
- Synthetic biology, enabling the design of artificial cells or signaling circuits that communicate through ions.
- Biomedical engineering, potentially leading to custom channels for sensors or diagnostic devices.
Because these new proteins are simpler and more stable than their natural counterparts, they may also be easier to produce and manipulate in laboratory settings. This opens the door to controlled studies of ion selectivity and membrane dynamics that were previously out of reach.
Honoring a Legacy of Ion Channel Research
This project also carries a personal note. The late William A. Catterall, a world-renowned ion channel researcher from the University of Washington, co-mentored Yulai Liu and contributed to this study before his passing. Catterall’s lifetime of work helped reveal how ion channels control nerve and heart activity and how their malfunction can lead to diseases such as epilepsy, autism, and cardiac arrhythmias. His expertise in electrophysiology was instrumental in guiding the testing of the designed proteins.
Looking Ahead
The next goal for the researchers is to expand their design methods to other metal ions, such as magnesium or potassium, to better understand how selectivity arises from atomic geometry and charge. They also aim to develop channels that can be switched on or off by external cues, adding new layers of control for experimental biology and medical applications.
This work illustrates the growing power of AI in molecular design — moving from theoretical modeling to the creation of entirely new biological functions. The ability to custom-build such complex, membrane-spanning proteins suggests that scientists are beginning to write the rules of life’s hardware, molecule by molecule.
While there’s still a long way to go before synthetic ion channels find medical or industrial use, this study demonstrates that the fundamental physics and chemistry of life can be decoded and rebuilt. It’s not just about copying nature anymore — it’s about understanding it well enough to redesign it.
Reference
Research paper: Bottom-up design of Ca²⁺ channels from defined selectivity filter geometry, Nature (2025)