CERN’s New Ion Trap Technique Could Reveal the Secrets of the Heaviest Elements
A team of scientists working at CERN’s ISOLDE facility has achieved something remarkable: they’ve developed a new experimental method that can measure the electron affinity of atoms with far fewer particles than ever before. This breakthrough could finally open a path toward studying the superheavy elements—those at the far edge of the periodic table that have so far remained mysterious due to their extreme instability and rarity.
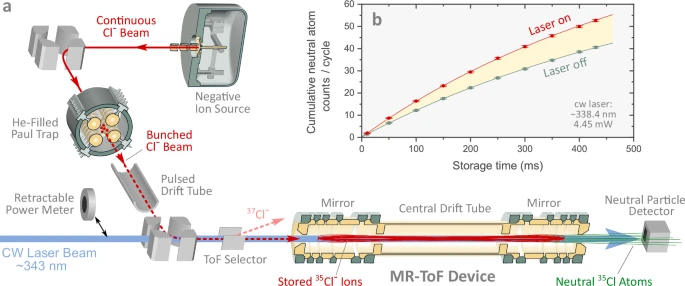
At the heart of this development lies a clever device called the Multi-Ion Reflection Apparatus for Collinear Laser Spectroscopy (MIRACLS). Instead of letting ions pass through a laser beam just once, as in traditional experiments, the ISOLDE researchers managed to “recycle” the same ions thousands of times. By bouncing them back and forth between electrostatic mirrors, they allowed the laser beam to probe them repeatedly, dramatically increasing the sensitivity of their measurements.
Understanding What the Scientists Measured
The team focused their demonstration on chlorine anions—negatively charged chlorine atoms. What they were trying to determine is known as electron affinity, which is the amount of energy released when a neutral atom gains an extra electron to become an anion. This is a fundamental property of every element, directly linked to how atoms bond together to form molecules and materials.
In a standard experiment, a beam of anions is sent through a laser beam. The laser’s frequency is adjusted until the photons have just enough energy to knock off the extra electron from the anion. The exact point at which this happens gives the electron affinity. But this traditional single-pass setup has a major limitation: when you’re studying superheavy elements, there simply aren’t enough ions produced to get a clear measurement. These rare atoms often exist for mere fractions of a second and are created at a rate of only a few ions per second—or even less.
The MIRACLS method solves this issue by trapping the ions inside an electrostatic cage where they bounce back and forth like ping-pong balls. Each time they pass through the laser beam, they can be probed again. In the chlorine experiment, the ions were reflected around 60,000 times, giving researchers a much longer interaction time. This recycling effect means that even with 100,000 times fewer ions, the precision matched that of traditional techniques.
Why This Matters for Superheavy Elements
Superheavy elements—those with atomic numbers greater than uranium’s 92—sit in a region of the periodic table where the rules of chemistry start to bend. As the number of protons increases, the electrons move faster and their behavior begins to show relativistic effects, meaning that Einstein’s theory of relativity starts to influence atomic structure.
Because of this, the normal periodic trends we learn in chemistry textbooks may not hold up for these elements. The structure of the electron shells could shift, and properties such as electron affinity, ionization energy, and bonding behavior might not follow predictable patterns. Some scientists even suggest that the boundaries between groups in the periodic table might begin to blur.
By using their ion-recycling trap, researchers at ISOLDE can now attempt to measure the electron affinities of these exotic elements, helping to confirm or challenge theoretical predictions. These measurements will be essential in understanding how the periodic table evolves at its upper limits and whether entirely new types of chemistry might emerge beyond it.
How the MIRACLS Trap Works
The MIRACLS setup is an elegant combination of precision engineering and clever physics. The trap contains two electrostatic mirrors—devices that reflect charged particles using electric fields rather than physical barriers. The ions are injected between the mirrors and oscillate back and forth many thousands of times.
Each time they pass through the laser beam, the experiment gathers more data on their interaction with photons. Because the same ions can be used repeatedly, researchers save enormous amounts of material, which is crucial when only a few atoms of a superheavy element may exist at any given moment.
The chlorine experiment was an ideal test case. Chlorine is a stable, well-understood element, making it perfect for checking whether the new method could reproduce known results. The outcome was impressive: the measured electron affinity matched the best previous results, proving that the MIRACLS technique works as intended.
Beyond Basic Chemistry – Potential Applications
While the immediate focus of the research is on fundamental physics and elementary chemistry, this technique could have practical implications in other areas. Measuring electron affinities precisely can improve theoretical models used to describe how atoms and molecules interact. This kind of data feeds directly into calculations used in nuclear medicine, antimatter research, and radioactive molecule studies.
One exciting example involves actinium and astatine, two rare elements that are being explored for targeted cancer therapies. These elements emit radiation that can destroy cancer cells when correctly attached to molecules that deliver them to tumors. Understanding their chemical properties in more detail—especially how easily they form negative ions—could help researchers design more effective and safer treatments.
Additionally, the MIRACLS approach could be extended to measure molecular electron affinities as well. That would allow scientists to verify and refine computational models used to simulate molecular structures and reactions. Such improvements ripple through fields as diverse as quantum chemistry, pharmaceutical design, and materials science.
The Bigger Picture – ISOLDE and the Quest to Understand Matter
The ISOLDE facility at CERN (the European Organization for Nuclear Research) is one of the world’s leading laboratories for studying exotic nuclei and rare isotopes. Researchers there specialize in producing and investigating radioactive and unstable forms of elements that can’t be found naturally on Earth.
This new development fits perfectly into ISOLDE’s mission. By enabling measurements with tiny quantities of atoms, MIRACLS allows the facility to study elements and isotopes that were previously beyond experimental reach. This not only strengthens the link between experiment and theory but also helps refine nuclear models that describe how matter behaves at the atomic level.
The MIRACLS project is a collaborative effort involving institutions such as the University of Greifswald in Germany, where the electrostatic ion mirror system was initially developed. Over the years, the setup has been optimized and integrated into CERN’s infrastructure to handle the unique challenges of working with radioactive isotopes and superheavy elements.
Looking Ahead
The success of the chlorine test is just the beginning. The next step is to apply the MIRACLS technique to rare and radioactive elements produced at ISOLDE. Each successful measurement adds a piece to the puzzle of how atomic structure evolves as we move toward the heaviest known elements.
Understanding these extreme atoms could eventually lead to the discovery of entirely new regions of stability on the periodic table, sometimes called the “island of stability”, where superheavy nuclei might live longer than a fraction of a second. If scientists can reach those regions, we might be able to study their chemistry in detail and even explore whether new types of matter could exist.
This method, by extending the limits of what can be measured, offers one of the most promising tools for exploring this frontier of science. From pure chemistry to medical innovation, the ability to study an element’s electron affinity using so few atoms could change the way we approach experiments with scarce or unstable materials.
Conclusion
CERN’s new ion-recycling trap represents a leap forward in how scientists can measure the most basic properties of atoms. By turning a simple idea—reusing ions through electrostatic reflection—into a practical and highly precise instrument, the researchers at ISOLDE have created a tool capable of shining light on the chemistry of the heaviest and rarest elements known to science.
The experiment demonstrates not only impressive technical mastery but also a vision for how future physics and chemistry can be done: using smarter, more efficient methods that extract maximum information from minimal material. In the coming years, as MIRACLS is applied to heavier and rarer elements, we can expect it to deepen our understanding of the building blocks of the universe in ways that were once thought impossible.
Research reference: F. M. Maier et al., “Enhanced sensitivity for electron affinity measurements of rare elements,” Nature Communications (2025)