A Protein Modification That Keeps Cells in Balance: How UFMylation of BiP Maintains ER Homeostasis
When cells make proteins, they rely on a special structure called the endoplasmic reticulum (ER) to fold those proteins into the correct shapes. If proteins don’t fold properly, they can pile up in the ER, stressing the cell and triggering a response known as the unfolded protein response (UPR). This stress, if unresolved, contributes to major diseases like type 2 diabetes, cancer, and neurodegenerative disorders.
A new study published in The FASEB Journal by Zhu Li and colleagues from Hangzhou Normal University and the University of South China dives deep into how a specific biochemical process—called UFMylation—helps cells handle this stress. The research reveals that UFMylation modifies a crucial protein known as BiP (also called GRP78 or HSPA5), ensuring the ER remains stable and functional. When this modification is disrupted, cells fail to manage stress and may die.
Understanding the Basics: The Role of BiP and the ER
About one-third of all proteins made in a cell pass through the endoplasmic reticulum, where they fold into their proper 3D structures. To keep this process orderly, the ER relies on three sensor proteins that monitor protein folding.
Under normal circumstances, BiP acts as a guardian—it binds to these sensors to prevent them from clumping together or activating unnecessarily. BiP also binds to unfolded or misfolded proteins to keep them from forming aggregates.
When too many misfolded proteins build up, BiP releases its grip on the sensors. This allows them to cluster, activating the unfolded protein response (UPR), which works to restore balance in the ER by halting protein production, refolding misfolded proteins, and increasing protein degradation.
This process of balance is what scientists call ER homeostasis—and BiP plays a central role in keeping it intact.
What Is UFMylation?
UFMylation is a relatively new type of post-translational modification—a biochemical tweak that changes how proteins behave after they’re made. It involves attaching a small molecule called UFM1 (Ubiquitin-Fold Modifier 1) to other proteins.
UFM1 works much like ubiquitin, another well-known protein modifier, but the two systems serve different purposes. While ubiquitination usually marks proteins for degradation, UFMylation often fine-tunes a protein’s stability, function, or location.
The UFMylation system involves a chain of enzymes:
- UBA5 (E1 enzyme) starts the process by activating UFM1.
- UFC1 (E2 enzyme) carries UFM1 to its next partner.
- UFL1 (E3 ligase) attaches UFM1 to the target protein, sometimes with the help of DDRGK1, which acts as a scaffold on the ER membrane.
This system has been increasingly linked to processes like cellular stress responses, autophagy, and protein quality control—but its role in maintaining ER balance wasn’t fully understood until now.
The Study: How UFMylation Controls BiP
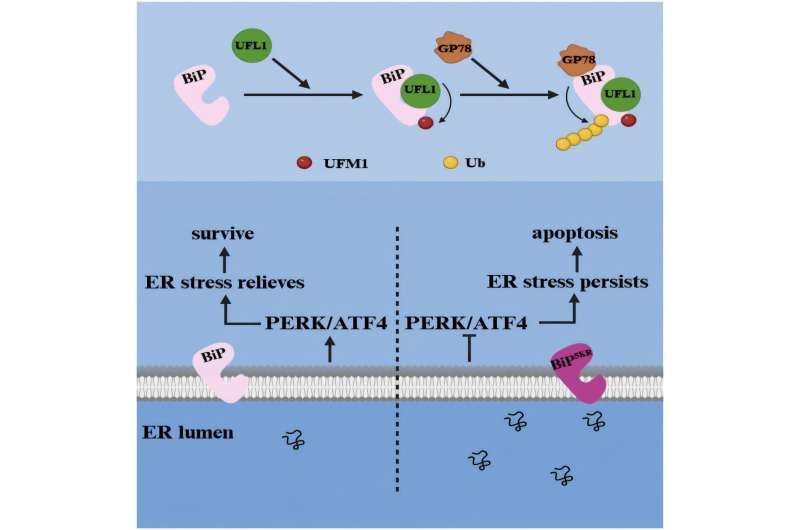
In this new research, the team led by Yu-Sheng Cong discovered that BiP can be UFMylated at five specific lysine residues: Lys294, Lys296, Lys352, Lys353, and Lys370. This was demonstrated through a series of experiments using immunoprecipitation and Western blot analyses in human cell lines.
When these five lysine sites were mutated so that UFM1 could no longer attach (creating a BiP5KR mutant), the UFMylation process was completely blocked.
The findings showed that UFMylation of BiP causes the protein to become less stable. UFMylated BiP is tagged for destruction through a partnership with another enzyme system—GP78-mediated ubiquitination. This means that UFMylation doesn’t just change BiP—it helps control its lifespan in the cell.
When UFMylation enzymes like UFL1 were reduced, or when BiP couldn’t be UFMylated (as in the BiP5KR mutant), BiP levels rose because it was no longer being degraded as quickly.
How Stress Changes the Equation
When the ER is under stress—such as when it’s flooded with misfolded proteins—things shift dramatically.
The researchers exposed cells to ER stress-inducing compounds like thapsigargin (TG) and tunicamycin (TM). They found that under these stressful conditions, the overall level of UFMylation-related enzymes actually went up. However, paradoxically, the binding between UFMylation enzymes and BiP went down, meaning BiP received fewer UFM1 modifications.
This change had a key effect: BiP became more stable. Rather than being degraded, it stuck around longer to help deal with the buildup of misfolded proteins.
This makes sense biologically—under stress, the cell needs more BiP to refold proteins and to help restore balance in the ER. By reducing UFMylation during stress, the cell effectively preserves BiP when it’s most needed.
When UFMylation Fails: The Consequences
To understand what happens when BiP cannot be UFMylated, the researchers looked at cells expressing the BiP5KR mutant. These cells behaved very differently under prolonged stress.
Normally, during long-term ER stress, BiP should detach from the stress sensors, allowing the PERK/ATF4 signaling axis of the UPR to activate and help cells survive. But in the mutant cells, BiP stayed stuck to the sensors and failed to activate the PERK/ATF4 pathway.
As a result, ER stress persisted, and the cells eventually underwent apoptosis, or programmed cell death.
The conclusion: UFMylation of BiP is essential for properly activating the unfolded protein response and preventing ER stress-induced cell death.
Why This Matters
The study positions BiP as a new and direct substrate for UFMylation, showing that this modification acts like a switch to balance BiP’s stability and the ER’s ability to respond to stress.
Under normal conditions, UFMylation helps regulate BiP levels by promoting its degradation.
Under stress conditions, the cell reduces BiP UFMylation, stabilizing the protein to better manage unfolded proteins.
This adaptive mechanism helps maintain ER homeostasis, ensuring that cells can recover from stress rather than die from it.
Broader Implications for Disease and Therapy
ER stress plays a role in many serious diseases:
- Type 2 diabetes: where pancreatic cells lose their ability to manage misfolded insulin.
- Neurodegenerative diseases: such as Alzheimer’s or Parkinson’s, where misfolded proteins accumulate in neurons.
- Cancer: where tumor cells often exploit ER stress pathways to survive in harsh environments.
Understanding the BiP-UFMylation pathway opens up a new direction for medical research. If scientists can learn to control or mimic UFMylation, it might be possible to either protect cells (in diseases like diabetes or neurodegeneration) or make them more vulnerable (in cancer therapy).
Future work will likely explore how stress signals regulate UFMylation, why UFMylation of BiP drops during stress despite higher enzyme levels, and how this mechanism plays out in living organisms rather than just cell cultures.
Beyond BiP: The Expanding World of UFMylation
UFMylation isn’t limited to BiP. Other studies have linked it to ribosome quality control, DNA damage repair, immune regulation, and autophagy. Loss of UFMylation components like UFL1 or DDRGK1 can trigger ER stress and apoptosis in various tissues.
Interestingly, recent work has connected UFMylation to neurodegenerative conditions such as Alzheimer’s disease. Abnormal UFMylation activity has been observed in models of protein misfolding disorders, suggesting it may be a common thread linking multiple stress-related diseases.
The study by Li and colleagues adds an important piece to this puzzle by showing exactly how UFMylation influences one of the ER’s most vital players—BiP.
In Summary
- BiP keeps the ER balanced by preventing misfolded proteins from accumulating.
- UFMylation, the attachment of UFM1 to BiP, normally controls how much BiP is degraded.
- During ER stress, UFMylation of BiP decreases, stabilizing it so it can help the cell recover.
- When BiP cannot be UFMylated, cells fail to activate the PERK/ATF4 stress pathway and die from prolonged ER stress.
- These findings make BiP’s UFMylation a critical mechanism for ER homeostasis, with major implications for understanding and treating diseases linked to protein misfolding.
Research Reference:
Zhu Li, Qian Liang, Yanmin Guo, Yaxiang Wang, Miao Wang, and Yu-Sheng Cong. UFMylation of BiP/GRP78 Is Crucial for Maintenance of Endoplasmic Reticulum Homeostasis. The FASEB Journal (2025). DOI: 10.1096/fj.202500976RR





