Ancient Viruses Help Bacteria Fight Modern Phage Attacks – A New Layer of Microbial Defense Revealed
For billions of years, bacteria and viruses have been locked in an endless arms race. Bacteria evolve new ways to protect themselves, while viruses evolve clever ways to break through those defenses. Now, a team of researchers at Pennsylvania State University, led by Professor Thomas K. Wood, has discovered an astonishing bacterial defense system that turns ancient, inactive viruses into weapons against modern viral invaders.
Their study, published in Nucleic Acids Research (2025), reveals that bacteria can use the remnants of prehistoric viruses—known as cryptic prophages—to detect and block new viral infections. The work opens exciting possibilities for biotechnology, medicine, and even the food industry.
How Ancient Viruses Became Bacterial Bodyguards
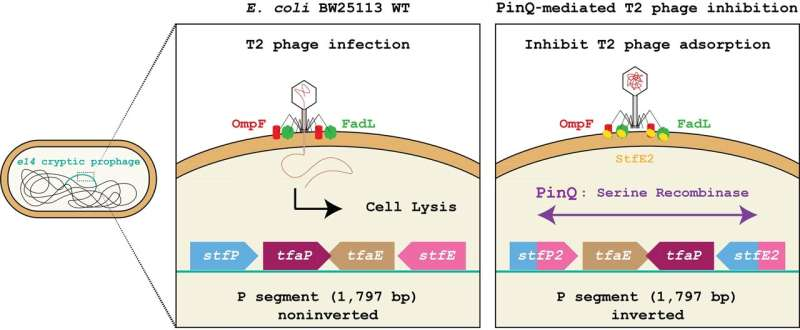
The research centers on a bacterial species that most people know well: Escherichia coli (E. coli). In its genome, E. coli carries traces of long-dead viruses that integrated into its DNA millions of years ago. These viral fossils are called cryptic prophages because they no longer produce active viruses—but some, it turns out, are far from useless.
Wood’s team discovered that one such prophage, known as Qin, contains an enzyme called PinQ, a type of serine recombinase. When an active virus—specifically the T2 bacteriophage—tries to infect the bacterium, PinQ suddenly activates. The enzyme cuts and flips a segment of DNA inside another prophage, called e14, located elsewhere in the bacterial genome.
This flipped section of DNA, known as the P segment, is about 1,797 base pairs long. When inverted, it generates two completely new chimeric proteins—named StfP2 and StfE2—that don’t exist in the normal orientation of the genome. Of these, StfE2 is the key defender. It blocks the invading virus from latching onto the bacterial cell surface, effectively stopping the infection before it begins.
Breaking Down the Mechanism
Here’s how this defense plays out step by step:
- Virus Detection: When E. coli senses the presence of phage T2, the PinQ enzyme is produced at about 14 times its normal level.
- DNA Inversion: PinQ then flips the P segment of the e14 prophage DNA within just a couple of hours.
- New Proteins Formed: The inversion creates StfE2, a novel protein that changes the bacterial surface in a way that prevents viruses from attaching.
- Defense Activated: The adsorption rate (virus binding) drops from nearly 100% in normal conditions to just 6–10% in bacteria expressing PinQ.
- Phage Mutations: After several infection rounds, some viruses evolve changes in their gp38 gene (which codes for a surface-binding protein), allowing them to bypass the defense—a reminder that the evolutionary arms race never ends.
- Reversal: When there’s no viral threat, the DNA flips back to its original state—suggesting the bacteria only deploy this defense when needed.
The results were confirmed using both laboratory experiments and computer modeling, which simulated how phages attempt to attach to bacterial receptors such as FadL and OmpF. The models and lab tests agreed: StfE2 interferes directly with viral attachment, without reducing receptor expression.
Even more striking, bacteria with active PinQ survived phage infection 400 times better than control cells, and produced 20 times fewer free viruses after infection.
Why This Discovery Matters
This finding adds a brand-new entry to the list of bacterial defense systems, which already includes well-known examples like restriction-modification, CRISPR-Cas, and abortive infection mechanisms. But this one stands out because it recycles ancient viral DNA—the remnants of infections that happened millions of years ago—and transforms it into a sophisticated, fast-acting antiviral system.
It’s like discovering that an old, rusted sword buried in the ground can suddenly sharpen itself and defend its owner again. This kind of built-in viral reprogramming hints at how flexible bacterial evolution can be.
Implications Beyond the Lab
1. Fighting Antibiotic Resistance
As antibiotic resistance continues to rise worldwide, scientists are looking at phage therapy—using viruses that target bacteria—as an alternative. However, before we can safely and effectively use phages to treat infections, it’s crucial to understand how bacteria naturally defend themselves.
This study provides exactly that kind of insight. If we know which bacterial genes or enzymes can stop phages, researchers can design more effective phage treatments or engineer bacteria to cooperate rather than fight the therapeutic viruses.
2. Applications in Food and Biotechnology
Phage attacks are also a major headache in the food industry. Many bacteria used in cheese, yogurt, and other fermented foods are vulnerable to viral contamination, which can ruin entire production batches. By understanding systems like PinQ-StfE2, scientists could engineer bacterial strains that are naturally resistant to such infections—saving both time and money.
3. The Evolutionary Perspective
This research beautifully illustrates evolution in action. It shows how bacteria can turn once-harmful viruses into permanent, beneficial components of their DNA. The Qin and e14 prophages are like genetic fossils that bacteria have learned to repurpose for survival.
In a broader sense, it reminds us that viruses are not just agents of disease—they are also major drivers of evolution. Their interactions with cellular life have shaped genomes for billions of years, leading to unexpected innovations like this defense mechanism.
The Broader Context: Cryptic Prophages and Recombinases
Cryptic prophages are common in bacterial genomes. Though they no longer form active viruses, many contain functional genes that can help the host cell in surprising ways—improving stress tolerance, enhancing metabolism, or, as shown here, protecting against new infections.
Recombinases like PinQ are enzymes that cut and rejoin DNA strands. They are used widely in genetic engineering because of their precision, but in nature, they serve diverse roles—from gene rearrangement to phase variation in pathogens. The fact that this recombinase helps defend bacteria highlights how multifunctional such enzymes can be.
Interestingly, the PinQ gene itself lies dormant under normal conditions. It only becomes active when a virus attacks, suggesting that bacteria may have evolved sophisticated sensing mechanisms to detect viral stress signals—though exactly how the activation is triggered remains an open question.
Challenges and Future Work
The Penn State team plans to study eight more cryptic prophages in their lab to see if similar defense mechanisms exist. It’s possible that many other bacteria are hiding comparable systems—genetic time bombs that activate under viral threat.
However, questions remain:
- How does the bacterium sense the incoming virus before activating PinQ?
- What is the exact structure and function of the StfE2 protein?
- How much energy or fitness cost does this system impose when active?
- Could this mechanism be transferred to other bacteria for industrial use?
While more research is needed, this discovery adds another chapter to the ongoing story of bacterial ingenuity. The ancient viral fossils within bacterial DNA aren’t just relics—they’re active participants in modern microbial survival.
Final Thoughts
This study gives us a glimpse into nature’s resourcefulness. Old viruses, once the enemies of bacteria, have become their allies. The PinQ-based system in E. coli proves that even genetic leftovers can evolve into powerful defense tools when the pressure of survival demands it.
As researchers continue to uncover similar systems, we’re likely to find that bacteria—and the ancient viruses within them—still have many tricks left to show us.
Research Paper: Adsorption of phage T2 is inhibited due to inversion of cryptic prophage DNA by the serine recombinase PinQ – Nucleic Acids Research (2025)





