Automated Chloroplast Platform From Max Planck Scientists Could Revolutionize Crop Engineering

A team of researchers at the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, has created a groundbreaking automated platform that could drastically speed up the development of improved crops. The system allows scientists to engineer and analyze thousands of chloroplast variants in parallel, potentially changing how we design plants for better productivity, stress tolerance, and climate resilience.
Chloroplasts—the light-powered factories inside plant cells—are responsible for photosynthesis and house several critical biochemical pathways. For years, scientists have dreamed of harnessing chloroplasts to engineer plants with superior traits, from higher yield to better carbon capture. But progress in chloroplast biotechnology has been slow because existing tools weren’t suited for fast, large-scale experimentation. That’s exactly what this new “Turbo Platform for Plant Research” promises to fix.
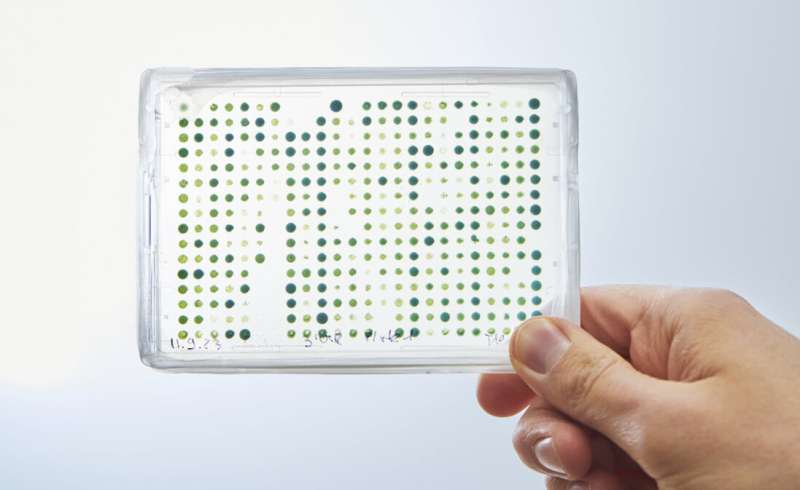
Credit: MPI for Terrestrial Microbiology / Gina Bolle
Breaking the Speed Barrier in Chloroplast Engineering
Until now, modifying chloroplast DNA has been a laborious, low-throughput task. Plant biotechnologists could only test a handful of genetic variations at a time, making it difficult to find the optimal combinations of genes or regulatory elements. The Max Planck team, led by Tobias Erb and carried out by researcher René Inckemann, has overcome this hurdle with a micro-algal test system built around Chlamydomonas reinhardtii, a well-studied green alga.
This system lets scientists perform rapid, automated testing of thousands of genetic constructs directly in chloroplasts. Using microtiter plates and robotics, the researchers can now generate and screen thousands of transplastomic algal lines—organisms whose chloroplast genomes have been altered—with unprecedented speed.
The process is also fully compatible with standardized biotechnology systems, such as modular DNA assembly frameworks, meaning the same genetic parts can be shared and reused easily between labs. The platform supports automation, standardization, and scalability, making it possible to explore chloroplast biology in a systematic, data-driven way—something that wasn’t feasible before.
What Makes Chloroplasts So Valuable for Bioengineering
Chloroplasts are special because they have their own DNA, separate from the cell’s nucleus. That means scientists can insert genes directly into the chloroplast genome, which offers several advantages:
- Stable integration: Inserted genes tend to stay in place, avoiding the random integration that happens with nuclear DNA.
- Lower risk of gene escape: Chloroplasts are usually inherited maternally, so there’s less chance of modified genes spreading through pollen.
- High protein production: Chloroplasts can express large amounts of foreign proteins, useful for producing enzymes, biopharmaceuticals, or industrial compounds.
Despite these advantages, the field of chloroplast biotechnology has lagged behind other areas of synthetic biology because it lacked a way to test and optimize designs quickly. The new platform removes that barrier by introducing high-throughput automation directly at the chloroplast level.
Testing Over 140 Genetic Parts
One of the most impressive aspects of the new system is the library of genetic regulatory parts the researchers developed and tested. In total, they characterized over 140 DNA elements that control gene expression in chloroplasts—including promoters, 5′ and 3′ untranslated regions (UTRs), and intercistronic expression elements (IEEs). These parts span a wide range of expression strengths, allowing scientists to fine-tune the output of each gene precisely.
This comprehensive mapping means that for the first time, biologists can design genetic circuits inside chloroplasts with predictable results. The library follows biotechnology standards that make it interoperable with widely used cloning and assembly systems, ensuring that other labs can easily adopt it.
Plant scientist Felix Willmund from the neighboring Center for Synthetic Microbiology (SYNMIKRO) has already begun using the technology to study and develop more robust chloroplasts, demonstrating its immediate practical potential.
Doubling Algal Biomass: Proof of Concept
To show that the system works, the researchers introduced a synthetic metabolic pathway into the chloroplasts of Chlamydomonas reinhardtii. This engineered pathway enhanced the alga’s ability to capture and utilize carbon dioxide under stress conditions. The result was remarkable: the modified algae produced almost twice as much biomass compared to unmodified ones.
This experiment serves as proof that the platform can identify and optimize genetic modifications that significantly improve biological performance. Once validated in the algal model, the most promising designs can be transferred into crop plants, reducing the time and cost from concept to field trial. That makes this platform a potential game-changer for agricultural innovation.
From Algae to Crops
The researchers emphasize that while the current work is done in algae, the approach is directly relevant to crop improvement. By testing thousands of chloroplast modifications in microalgae first, scientists can select only the best candidates for more complex plant systems. This pipeline approach accelerates the entire process—from molecular design to greenhouse trials.
Potential applications include:
- Improving resilience to heat, drought, and excess light
- Enhancing nutrient content and photosynthetic efficiency
- Increasing yield and growth rate
- Developing new carbon-fixation routes
- Producing high-value compounds such as pharmaceuticals or nutritional supplements
By providing a solid foundation for chloroplast engineering, the platform could open the door to climate-smart agriculture and bio-based manufacturing of valuable molecules.
Collaboration and Climate Relevance
The work is part of larger research initiatives like Robust Chloroplast and the Excellence Cluster Microbes-4-Climate, in collaboration with Marburg University. These programs aim to develop biological solutions to climate challenges by using microbes and plants to capture carbon more effectively and produce sustainable materials.
The new chloroplast screening platform aligns perfectly with this vision. As the climate crisis intensifies, speeding up research on biological carbon fixation and plant resilience is essential. Tools that enable rapid experimentation, like this one, allow science to keep pace with the urgency of the problem.
The Bigger Picture: Synthetic Biology Meets Photosynthesis
This development also reflects a broader trend in synthetic biology—the effort to redesign living systems for useful purposes. Until recently, most high-throughput synthetic biology work was limited to bacteria and yeast. Plant systems were too slow and complex for large-scale automation. By adapting those methods to chloroplasts through an algal model, the Max Planck team is bridging that gap.
Algae like Chlamydomonas reinhardtii grow quickly, are easy to transform genetically, and share many photosynthetic features with plants. This makes them ideal for testing new ideas before applying them to crops. Once the best genetic configurations are identified, they can be inserted into the chloroplast genomes of plants like rice, maize, or wheat for real-world agricultural applications.
Such cross-platform approaches could make plant engineering as agile as microbial engineering—a long-standing goal in the field.
Challenges Ahead
While the new system represents a major leap, there are still challenges to overcome before its benefits reach farmers. Translating successful algal results to complex crops involves differences in chloroplast regulation, transformation efficiency, and growth environments. Large-scale field testing, regulatory approval, and biosafety considerations will also take time.
Nonetheless, this breakthrough offers a clear roadmap for how future plant biotechnologists might work: test fast in algae, optimize with data, and move the best candidates into crops for real-world impact. It’s a much faster and more efficient way to innovate.
Why This Matters Globally
As the world faces climate change, food insecurity, and declining agricultural productivity, innovations like this automated chloroplast platform offer a glimpse of hope. By making plant bioengineering faster and more systematic, scientists can explore new ways to feed a growing population, develop sustainable bio-based materials, and enhance environmental resilience—all powered by the natural efficiency of photosynthesis.
This technology could help researchers identify climate-resilient crop varieties and carbon-efficient plants, both of which are urgently needed for sustainable agriculture in the 21st century.
Research Reference:
René M. Inckemann et al., A modular high-throughput approach for advancing synthetic biology in the chloroplast of Chlamydomonas, Nature Plants (2025). DOI: 10.1038/s41477-025-02126-2