Detecting Ancient Proteins: A New Staining Technique Reveals Collagen Hidden in Fossils
Researchers from Okayama University of Science (OUS), Japan, have developed a groundbreaking way to visualize ancient proteins directly within fossils — a major leap forward for the field of paleoproteomics, which studies the remnants of ancient proteins to understand extinct organisms.
Their study, published in the Journal of Proteome Research (2025), introduces a modified histological staining method that allows scientists to see where proteins like collagen still exist inside fossilized tissues, without destroying the sample. This advancement could change how researchers verify the authenticity of ancient biomolecules, minimize contamination, and choose the best fossils for deeper molecular analysis.
Why Ancient Proteins Matter
Most people have heard about ancient DNA being extracted from fossils — like mammoth genomes or Neanderthal sequences. But DNA is fragile and often degrades completely after a few hundred thousand years. Proteins, on the other hand, are chemically more stable and can survive for millions of years.
That’s why paleoproteomics has become an exciting field: by studying ancient proteins, scientists can learn about evolutionary relationships, physiology, and even diseases of extinct animals. However, there’s a problem — most methods for analyzing proteins involve crushing fossils into powder to extract them.
While that might sound practical, it destroys the fossil’s microstructure and removes the protein from its original location. Worse, the process increases the risk of contamination from modern materials or microbes, making it difficult to confirm whether a detected protein truly came from the ancient organism or not.
This is where the new staining approach by the OUS team becomes valuable.
The New Method: Seeing Proteins in Place
The researchers, led by Hayato Inaba (graduate student and education specialist at the OUS Dinosaur Division) and Professor Hidetsugu Tsujigiwa, adapted a common biological staining method to visualize proteins inside fossils without demineralizing them.
Typically, when scientists prepare fossils for histology, they remove the minerals using acids — a process called demineralization. However, this can damage or distort the original tissue structure and make contamination more likely. The new method works on non-demineralized thin sections, keeping the fossil’s natural structure intact.
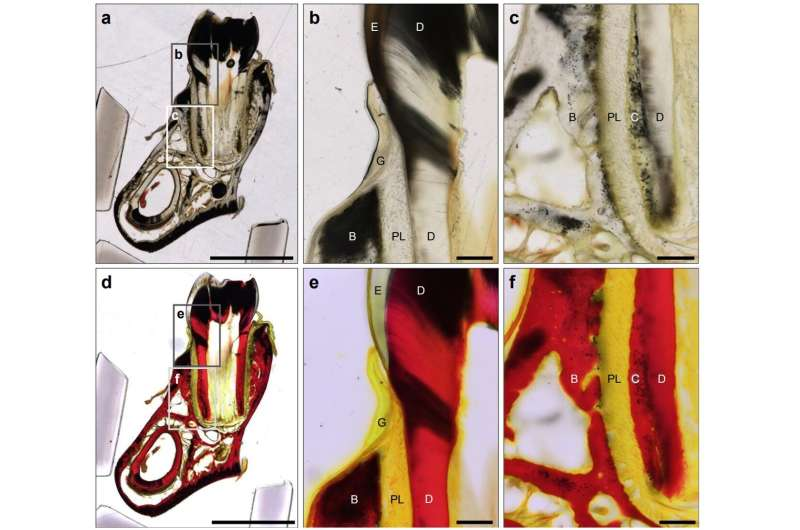
The OUS team tested several staining techniques on Pleistocene-aged elephant fossils (dating back tens of thousands of years) recovered from the Seto Inland Sea in Japan. Among the methods tested, Van Gieson’s staining turned out to be the most effective.
This classical stain — long used in modern biology — colors Type I collagen (the main structural protein in bone) a bright red, while other tissues appear yellow. When applied to fossil sections, it allowed the team to visually map the distribution of preserved collagen within the bone itself.
Confirming the Findings: Is It Really Ancient Collagen?
To make sure the red-stained material was indeed ancient collagen and not contamination, the team used state-of-the-art mass spectrometry. They analyzed the protein fragments extracted from the stained regions.
The results confirmed Type I collagen sequences that matched extinct elephants (Proboscidea), proving that the material was endogenous — meaning it originated from the ancient animal, not from modern sources.
Even more interestingly, the team discovered tissue-specific preservation differences. Fossilized bone retained collagen remarkably well, while dentin — the hard tissue that makes up tusks and teeth — showed almost none.
Why the difference? It turns out the microstructure of the tissues plays a big role. Dentin has thousands of tiny channels called dentin tubules that expose the inner matrix to environmental factors, accelerating protein decay and leaching over time. Bone, being denser, offers a more protective environment for collagen to survive through the ages.
This insight gives scientists an important guideline for future studies: focus on denser tissues like bone when searching for preserved proteins.
A Tool for the Future of Paleoproteomics
The simplicity and reliability of this staining method make it an ideal screening tool for researchers. Before committing to expensive and time-consuming mass spectrometry, scientists can now screen fossils visually to check whether collagen still exists in them — and where.
That’s a major efficiency boost for the field. It not only helps verify authenticity but also reduces the risk of false positives caused by contamination or environmental interference.
The OUS team plans to apply this approach to much older fossils, including those of dinosaurs, which are tens of millions of years old.
Extracting proteins from such ancient specimens is incredibly difficult because only trace amounts remain, but the researchers are hopeful. If they can eventually analyze the amino acid sequences from dinosaur proteins, it would allow scientists to go beyond morphological classification and study dinosaurs as living, physiological organisms — something that’s been out of reach until now.
What Is Van Gieson’s Staining?
For readers unfamiliar with histology, Van Gieson’s stain is a classic method used in biology and medicine to differentiate between collagen and muscle tissue in microscopic samples.
The stain uses a combination of picric acid and acid fuchsin. When applied, collagen fibers appear bright red, while cytoplasm and muscle turn yellow. This clear contrast makes it easy to see where connective tissues are located.
Adapting this technique for fossilized material was a bold move — fossils are mineralized and often brittle, so stains don’t always penetrate well. Yet the OUS team successfully modified the process so that the stain highlights preserved organic molecules even in fossil bones that have been buried for thousands of years.
Understanding Paleoproteomics in Context
Paleoproteomics is still a relatively young field but has already produced some incredible discoveries. Scientists have extracted collagen from 3.8-million-year-old ostrich eggshells, keratin from fossil feathers, and even blood vessel proteins from dinosaur bones under special conditions.
These proteins can tell us how ancient animals lived — their metabolism, immune systems, and evolutionary relationships — offering clues that pure fossil morphology cannot.
However, one of the biggest ongoing debates is about authenticity. Skeptics argue that many protein detections could be modern contamination — bacteria, fungi, or even human handling. Methods like this new in situ staining directly address that concern by showing where the proteins exist in the fossil microstructure. If the proteins are distributed within the tissue and aligned with the bone’s structure, it’s much more likely they are truly ancient.
Why This Study Matters
This development marks a major step forward in how scientists study ancient biomolecules. It doesn’t replace high-tech molecular analyses, but it provides a reliable, low-cost, and visual method for identifying promising samples.
By combining microscopy, histology, and proteomics, researchers now have a more complete toolkit for unlocking ancient biological information from fossils that were once thought to be chemically silent.
As Professor Tsujigiwa and colleagues suggest, this could bring us closer to viewing dinosaurs — and other extinct animals — not just as skeletons, but as organisms whose biochemistry can still be read, even after millions of years.
Reference
Research Paper: New Application of Histological Staining for Visualization of Endogenous Proteins in Fossil Material – Journal of Proteome Research (2025), Okayama University of Science, Japan.